More Information
Submitted: November 03, 2025 | Approved: November 06, 2025 | Published: November 07, 2025
How to cite this article: Alsalem M, Malas H, Raza G, Ismael R. Misoprostol Usage in the Kingdom of Bahrain: A Retrospective Study. Clin J Obstet Gynecol. 2025; 8(4): 092-095. Available from:
https://dx.doi.org/10.29328/journal.cjog.1001196
DOI: 10.29328/journal.cjog.1001196
Copyright license: © 2025 Alsalem M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Misoprostol Usage in the Kingdom of Bahrain: A Retrospective Study
Maryam Alsalem*, Hosni Malas, Gulmeen Raza and Rehab Ismael
Building 2435, Road 2835, Block 228, P.O Box 24343, Busaiteen, Kingdom of Bahrain
*Address for Correspondence: Maryam Alsalem, Building 2435, Road 2835, Block 228, P.O Box 24343, Busaiteen, Kingdom of Bahrain, Email: [email protected]
Introduction: The document introduces the topic of medical termination of pregnancy (MTP) using misoprostol, a synthetic prostaglandin E1 analogue. It discusses the advantages and challenges of this method, and the research gap in the Gulf region and the Middle East. It also states the aim of the study, which is to evaluate the safety, efficacy, and acceptability of misoprostol for MTP in Bahrain.
Methods: The document describes the study design, data source, and inclusion criteria. It explains that the data was collected from the Hope system in King Hamad University Hospital and included women in the first and second trimester of pregnancy who were undergoing MTP as per the FIGO protocol. It also mentions the statistical analysis that was used to compare the outcomes of different regimens, dosages, and routes of administration of misoprostol.
Results: The document reports the main findings of the study, such as the success and failure rates of MTP, the association between previous deliveries and MTP outcomes, the optimal number and route of misoprostol doses, the length of hospital stay, and the incidence of complications. It also presents some figures to illustrate the results.
Discussion: The document interprets the results and compares them with previous studies. It highlights the high efficacy of the sublingual route of misoprostol but also acknowledges the side effects and limitations of this route. It suggests that two doses of 600mcg or 800mcg of misoprostol are sufficient for MTP. It also identifies the strengths and weaknesses of the study and proposes some future directions for research.
Conclusion: The document concludes that the study provides a better understanding of the outcomes of misoprostol for MTP in a controlled environment. It asserts that misoprostol is a safe and effective option for women seeking abortion in the first and second trimester, especially in low-resource settings. It also emphasizes the need for evidence-based guidelines and counseling for misoprostol use.
Medical termination of pregnancy (MTP) is a common practice around the world, especially in countries where abortion is legal. Misoprostol alone is a highly effective method of pregnancy termination [1]. It is a synthetic prostaglandin E1 analogue, has been approved by the World Health Organization (WHO) as a safe and effective medication for MTP. It has a wide range of reproductive health applications, including induction of labor, management of spontaneous and induced abortion, and prevention and treatment of postpartum hemorrhage [2]. Misoprostol is affordable and can be administered even in low-resource settings, making it an attractive option for women seeking abortion. However, there are still gaps in knowledge within the Middle East regarding optimal regimens, dosages, and routes of administration for this medication, as well as the potential side effects and complications associated with its use.
Thirty years after misoprostol was first marketed in France in 1988 as a medication used to treat gastric ulcers, many questions about it have been answered in numerous clinical studies. It is also noted that misoprostol is effective and safe and is a reasonable option for women seeking abortion in the first trimester [3]. As of today, there are only a few clinical trials on misoprostol-alone regimens that use the previously endorsed regimen of 800-μg misoprostol tablets administered sublingually, vaginally, or buccally every 3 hours for at least 3 doses (2400 μg total). Unfortunately, the usage of misoprostol as monotherapy suffers from a severe lack of research in the Gulf region and the Middle East. For instance, research conducted in Saudi Arabia, one concerning finding was the users' poor knowledge about misoprostol, and that increased awareness about the inherent risks associated with its unsupervised use as an abortifacient in illegal abortions is needed [4].
Misoprostol usage in the Kingdom of Bahrain is strictly prohibited outside a hospital setting.
Patients needing access to misoprostol for medical termination must be admitted to the hospital.
This protocol, which is followed by all hospitals in the Kingdom of Bahrain, including King Hamad University Hospital, ensures that each patient receiving the medication is closely monitored, hence eliminating its misuse.
This research aims to evaluate the safety, efficacy, and patient’s acceptability of misoprostol for medical termination of pregnancy, with a focus on optimizing treatment protocols and minimizing risks. Furthermore, contribute to the development of an evidence-based guideline to improve the overall usage of misoprostol in the termination of pregnancy in the Kingdom of Bahrain. Since the usage of misoprostol in medical termination of pregnancy is not widely researched in the Middle East, this will aid in adding data to help physicians in the Gulf when it comes to counselling patients regarding the usage and outcome, which in turn will assist in overall patient satisfaction along with prognosis in the population of Bahrain.
This study is retrospective, data were collected from the electronic medical records in King Hamad University Hospital. This included women in the first and second trimester of pregnancy diagnosed as missed miscarriage and incomplete miscarriage who were undergoing medical termination or completion of abortion as per the FIGO protocol [5] (Figure 1A) between the timeframes of January 2022 and December 2024. With the SPSS program, the data collected will be analyzed to form a conclusion about the safety, efficacy, and acceptability of misoprostol in medical termination of pregnancy amongst one population.
Figure 1a: Medical termination of pregnancy per FIGO protocol 2017 (permission received to publish).
The patients in this study received misoprostol as per the FIGO protocol of 2017. If the patient gave a history of passage of tissue or blood, they are assessed by ultrasound to determine whether the medical termination was complete (i.e., successful), meaning that the endometrial thickness was less than 16mm with no evidence of an intrauterine gestational sac according to the definition provided by the Royal College of Obstetrics and Gynecologists. However, if the patient was hemodynamically unstable, had active per vaginal bleeding or that the ultrasound finding showed an endometrial thickness greater than 16mm with or without complete expulsion of the gestational sac then the medical termination was not complete (i.e. unsuccessful) and the patient would require surgical intervention.
Data analysis
Continuous variables are represented as Median (Interquartile range), whereas categorical variables are represented as frequencies and percentages. Depending on the data requirements, Chi-square test and Fisher’s exact test were used to assess associations between categorical variables. To test the difference of medians between groups, the Mann-Whitney U test for nonparametric variables was used. SPSS (version 26.0) was used to conduct all analyses. A p-value of less than 0.05 was considered statistically significant.
The analysis included a total of 169 patients of which 76 (45%) were classified as successful cases (complete abortion, not requiring surgical intervention), while 93 (55%) were not successful (incomplete abortion, requiring surgical evacuation of retained products of conception).
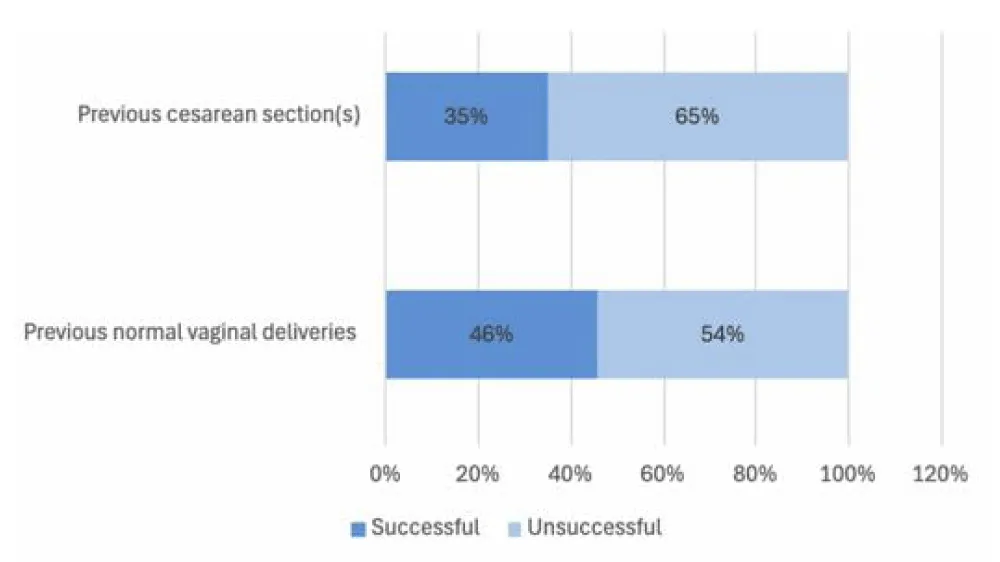
One-hundred patients out of the 169 had previous normal deliveries only, of those 100 the success rate was 46%. Thirty-nine patients had previous cesarean sections of which 14 had successful medical termination (35%), the comparison between the success rates is highlighted in Figure 1B. The failure rate in comparison was higher in those who had cesarean sections (64.1%) than those who had normal deliveries (54%) with the p-value of 0.023 (Figure 2A).
Figure 1b: Comparing the success rate of medical termination in patients who had previous vaginal deliveries to those who had previous cesarian sections.
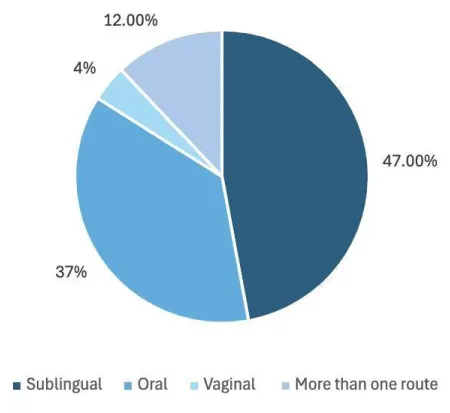
Figure 2a: Highlighting the routes of administration and their success.
The number of misoprostol taken had a statistically significant association with the outcome showing a p-value of 0.016. Patients who took 600mcg or 800mcg of misoprostol, meaning that had 3 or 4 tablets respectively every 3 hours, had the highest success rate when compared to others which was 50% (38 patients). Those who took 3 tablets of misoprostol made up 10% (8 patients) of the success rate, and those who received four tablets made up 2.6% (2 patients).
The sublingual route had the highest success rate, making up 36 patients (47%). Those who received oral misoprostol were next which included 28 of the 169 patients (37%). Nine patients had misoprostol via more than one route with a success rate of 12%. The least successful route was the vaginal route with only 3 patients having success (4.0%) (Figure 2A).
The length of hospital stay proved to be of significance in this study. Fifty-two patients who received medical termination had the shortest hospital stay, making up 68.4% and with the p-value of 0.003.
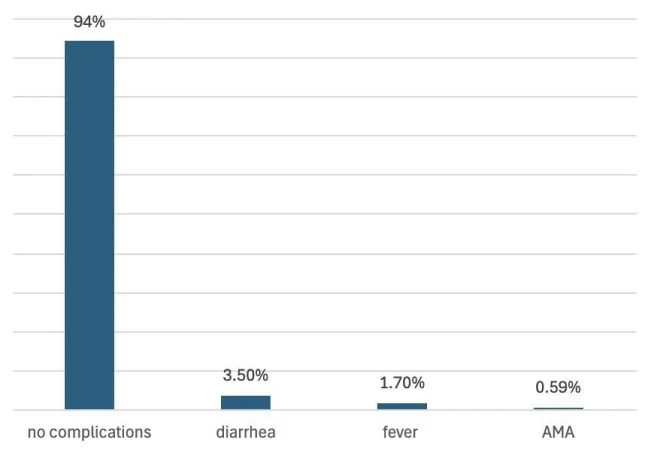
Ten patients (5.9%) who underwent medical termination had complications, 6 of them had diarrhea, 3 had experienced fever (two of which had a fever of 38 degrees, and one had 39.1), and only one patient had active vaginal bleeding that resulted in hypotension and was taken immediately for surgical evacuation. One patient refused continuation of medical termination and surgical evacuation, that patient signed against medical advice and left the hospital. Of those 10 patients, 4 of them had successful medical termination and the other 6 patients had incomplete miscarriage and had to undergo surgical evacuation of retained products of conception (Figure 2B). Only one patient refused total management, making the acceptance rate 99%.
Figure 2b: Shows the complications of medical termination using misoprostol.
In this study, we reviewed the efficacy of misoprostol in medical termination of missed abortion. The number of patients who received misoprostol spent less time within the hospital with very low complication rate. Only a few had complications (5.9%), however most of those patients had successful medical termination.
The sublingual route shows a higher success rate than the other routes of administration and the vaginal route having the least success rate unlike that reported by Wu, et al. [6] which stated that the least effective treatment was the oral misoprostol of 400 ug due to liver first-pass effect which greatly reduced the bioavailability of the medication. In another study by Khan, et al. [7] it is shown that the sublingual route had greater success than the vaginal route.
Although the sublingual route is said to be the most successful it is associated with more side effects than either oral or vaginal administration [8]. Six patients in this study had suffered from diarrhea, which is known to be the most frequently encountered side effect, which is self-limiting, due to misoprostol’s increase concentration in the plasma [9].
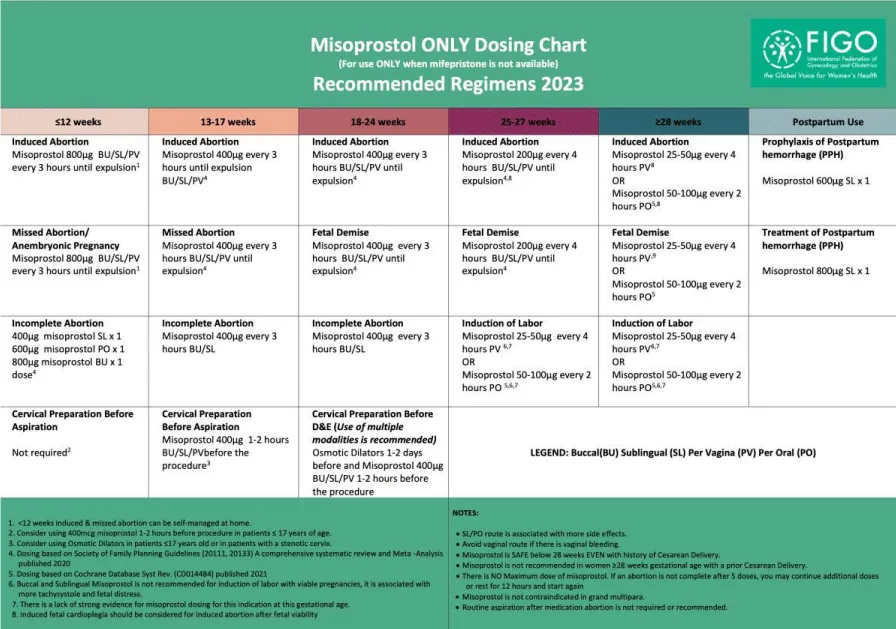
Patients who received only two doses of 600 µg or 800 µg of misoprostol, with an interval of 3 hours in between each dose yielded the most successful outcome and showed statistical significance which goes with the new FIGO protocol published in 2023 (Figure 3). In comparison to a systematic review that included 20 studies in which patients received at least 3 or more doses of 800 µg of misoprostol, 87% of the 5338 evaluated patients aborted completely without additional treatment [10].
Figure 3: Medical termination of pregnancy per FIGO protocol 2017 (permission received to publish).
The successes of this study included having easy access to data collection. The electronic medical system provided in King Hamad University Hospital allowed easy access to patients’ files including access to the medications received during hospital stay and the course of treatment. The limitation was that our study is a retrospective one.
The study itself showed that usage of misoprostol in a controlled environment as per the FIGO protocol yielded very few patients with complications. It also showed that those patients who underwent medical termination had short hospital stays. Patients that didn’t have successful outcomes were mainly those who received low doses of misoprostol at less frequent doses, which matches the new FIGO protocol published in 2023. It also confirms the effectiveness of misoprostol.
- Jayaweera R, Egwuatu I, Nmezi S, Kristianingrum IA, Zurbriggen R, Grosso B, et al. Medication abortion safety and effectiveness with misoprostol alone. JAMA Netw Open. 2023;6(10):e2340042. Available from: https://doi.org/10.1001/jamanetworkopen.2023.40042
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology, Society of Family Planning. Medication abortion up to 70 days of gestation: ACOG practice bulletin, number 225. Obstet Gynecol. 2020;136(4):e31-e47. Available from: https://doi.org/10.1097/aog.0000000000004082
- Raymond EG, Grossman D, Mark A, Upadhyay UD, Dean G, Creinin MD, et al. Commentary: No-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361–6. Available from: https://doi.org/10.1016/j.contraception.2020.04.005
- Alsibiani SA. Use of misoprostol for self-induced medical abortions among Saudi women: a call for attention. Gynecol Obstet Invest. 2014;78(2):88-93. Available from: https://doi.org/10.1159/000363238
- Morris JL, Winikoff B, Dabash R, Weeks A, Faundes A, Gemzell-Danielsson K, et al. FIGO's updated recommendations for misoprostol used alone in gynecology and obstetrics. Int J Gynecol Obstet. 2017;138:363-366. Available from: https://doi.org/10.1002/ijgo.12181
- Wu HL, Marwah S, Wang P, Wang QM, Chen XW. Misoprostol for medical treatment of missed abortion: a systematic review and network meta-analysis. Sci Rep. 2017;7:1664. Available from: https://doi.org/10.1038/s41598-017-01892-0
- Khan UI, Iftikhar B, Jabeen SS, Akram NA, Subktageen B, Fatima A. Comparison of the sublingual and vaginal misoprostol for medical termination of pregnancy in cases of first and second trimester abortion: a quasi-experimental study. Pak Armed Forces Med J. 2022;72(2):414-418. Available from: https://www.pafmj.org/PAFMJ/article/view/7241
- Hamoda H, Ashok PW, Flett GM, Templeton A. A randomized controlled comparison of sublingual and vaginal administration of misoprostol for cervical priming before first-trimester surgical abortion. Am J Obstet Gynecol. 2004;190:55–59. Available from: https://doi.org/10.1016/j.ajog.2003.08.025
- Turner JV, Agatonovic-Kustrn S, Ward H. Off-label use of misoprostol in gynaecology. Facts Views Vis Obgyn. 2015;7(4):261-264. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5058416/
- Raymond EG, Harrison M, Weaver MA. Efficacy of misoprostol alone for first-trimester medical abortion. Natl Libr Med. 2019. Available from: https://doi.org/10.1097/AOG.0000000000003017