More Information
Submitted: December 13, 2022 | Approved: June 12, 2025 | Published: June 13, 2025
How to cite this article: Sakthi A. Impact of Thin Endometrium in Frozen Embryo Transfer: Thesis Summary Article. Clin J Obstet Gynecol. 2025; 8(2): 037-056. Available from:
https://dx.doi.org/10.29328/journal.cjog.1001187
DOI: 10.29328/journal.cjog.1001187
Copyright license: © 2025 Sakthi A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Thin Endometrium; Frozen Embryo Transfer (FET); Endometrial Thickness (EMT); Endometrial Receptivity; Assisted Reproductive Technology (ART); Platelet-Rich Plasma (PRP); Granulocyte Colony Stimulating Factor (G-CSF); Angiogenesis
Impact of Thin Endometrium in Frozen Embryo Transfer: Thesis Summary Article
Sakthi A*
Department of Reproductive Medicine, Saveetha Institute of Medical & Technical Sciences, Thandalam, Chennai- 602105, India
*Address for Correspondence: Sakthi A, Department of Reproductive Medicine, Saveetha Institute of Medical & Technical Sciences, Thandalam, Chennai- 602105, India, Email: [email protected]
Background: Endometrial Thickness (EMT) is considered an important prognostic factor in assisted reproductive technology (ART), particularly in frozen embryo transfer (FET) cycles. Thin endometrium (< 7 mm) is often associated with implantation failure and reduced pregnancy rates, although its precise impact remains controversial.
Objective: This thesis summary evaluates the role of thin endometrium in FET cycles, discusses etiologies, diagnostic modalities, treatment approaches, and reviews available patient data.
Methods: We retrospectively analyzed 69 patients who underwent FET at Saveetha Medical College. Endometrial thickness was assessed via transvaginal ultrasound. Various treatment strategies including hormonal therapies (estradiol, progesterone), vasoactive agents, granulocyte colony-stimulating factor (G-CSF), platelet-rich plasma (PRP), L-arginine, sildenafil citrate, vitamin E, pentoxifylline, and stem cell therapy were reviewed for their efficacy in improving endometrial receptivity.
Results: Endometrial thickness < 7 mm correlated with lower pregnancy rates compared to patients with thickness ≥ 7 mm. Hormonal and adjuvant therapies reported varying degrees of success in improving EMT and subsequent pregnancy outcomes. Vascularity, angiogenesis, and Doppler studies also demonstrated a significant association with endometrial receptivity.
Conclusion: Thin endometrium remains a critical challenge in ART cycles. Multimodal therapeutic approaches may improve outcomes; however, larger prospective studies are required to establish standardized protocols. Our patient data support that even minimal increases in EMT can enhance implantation success.
Successful implantation requires high-quality embryos, a receptive endometrium, adequate vascularity, and perfect synchronization. The receptive endometrium define as a healthy uterine lining undergoing transformation of endometrial cells into decidual cells appropriate for implantation of blastocysts, and rapid growth of placenta. Therefore, endometrial assessment is routinely performed during in vitro fertilization (IVF) and intra cytoplasmic sperm injection (ICSI). In addition endometrial thickness (EMT) have been considered as a marker of endometrium receptivity and a prognostic factors for embryo transfer during IVF/ICSI treatment. Appropriate endometrial thickness is essential for achieving pregnancy and several studies have stated low pregnancy rates in patients with a thin endometrium. The optimal endometrial thickness for conception remains controversial among clinicians. EMT less than 7 mm on ultrasound is generally considered sub-optimal for embryo transfer and is correlated to a decreased probability of pregnancy.
The success of IVF cycles is mainly dependent on age, quality of the embryo, and endometrial receptivity. Endometrial receptivity is widely regarded as a key factor in the success of IVF. Also, the evaluation of endometrial receptivity has been the focus of interest for many years due to its potential clinical importance. Generally, ultrasound examination has been carried out as routine method of endometrium evaluation in IVF cycles in most reproductive medicine. Several sonographic parameters have been developed in the identification of endometrial receptivity, including endometrial thickness, endometrial pattern, endometrial volume, and endometrial and sub endometrial blood flow [1,2], among which endometrial thickness and endometrial pattern have been widely accepted as prognostic indicators for endometrial receptivity. However, there is still no consensus on whether the endometrial sonographic characteristics can predict the pregnancy outcome.
Endometrial features assessed included endometrial thickness and pattern on the day of progesterone supplementation in FET cycles. Endometrial thickness was measured in the midsagittal plane of the uterus as the maximum distance between the 2 interfaces of endometrial–myometrial junction [3]. Endometrial pattern were classified according to the morphology of the endometrium as: pattern A (triple-line type characterized by a hypoechoic Endometrium with well-defined hyperechoic outer walls and a central echogenic line); pattern B (isoechoic endometrium with poorly defined outer walls and central echogenic line); pattern C (homogeneous hyperechoic endometrium) [4]. Endometrial receptivity is referred to the ability of endometrium to accept and accommodate a blastocyst, resulting in the process of implantation.
The Uterus, which carries the fetus in females throughout the pregnancy, it has three layers innermost, middle and outer layer. The innermost layer of the uterus is called Endometrium. A thick and healthy endometrium is crucial for normal menstrual cycle and successful pregnancy [5]. A thick and healthy Endometrium, also known as Uterine Lining, goes a long way in ensuring effective implantation of fertilized eggs inside the uterus. Later, during the pregnancy, it also nourishes the fetus. If women are grappling with issues related to pregnancy, like unable to get pregnant or difficulty in holding a pregnancy, they must get their estrogen level and thickness of Endometrium checked.
Estrogen hormone is extremely vital in keeping Endometrium thick, salutary and blood-rich. Inadequate level of estrogen results in thin Endometrium. If a Uterine Lining or Endometrium is minimum 8 mm thick, it is considered satisfactory. Anything less than 7 mm is called Thin Endometrium.
The definition of a thin endometrium varies between studies, but is generally defined as < 7 or < 8 mm on the day prior to the start of progesterone in frozen–thaw embryo transfer (ET) cycles.
Endometrial receptivity can be induced by exogenous administration of estradiol and progesterone in various protocols for endometrial preparation. Estradiol priming contributes to endometrial proliferation and induction of progesterone receptors. Then, the action of subsequent progesterone turns the estrogen-primed endometrium into a secretory structure for embryo survival and implantation. Therefore, for those females with thinner endometrium in fresh cycles, it would be useful to prolong the duration of estradiol stimulation for optimal endometrial receptivity. For those females with thin endometrium in fresh cycles, additional estradiol stimulation might be helpful for adequate endometrial development.
Aim and objectives
To study about the outcome of patients with thin endometrium, in frozen FET. The thin endometrium < 7 mm, shows implantation failure or poor pregnancy outcome, with estradiol supplements, endometrial thickness and receptivity might be improved.
The endometrium is innermost lining of the uterus, playing key roles during the menstrual cycle as well as during pregnancy [6]. Also called the endometrial lining, the tissue it's made up of serves as the "mucosal lining of implantation" of the uterus, or womb ‘the pear-shaped organ that houses a developing fetus. Abnormalities of the endometrium can result in concerns such as endometritis, hyperplasia, and cancer.
The endometrium is the inner epithelial layer, along with its mucous membrane, of the mammalian uterus. It has a basal layer and a functional layer; the functional layer thickens and then is shed during menstruation in humans. During pregnancy, the glands and blood vessels in the endometrium further increase in size and number. Vascular spaces fuse to form the placenta, which supplies oxygen and nutrition to the embryo and fetus at the same time having endocrine, immunological and excretory functions.
Anatomy of Endometrium
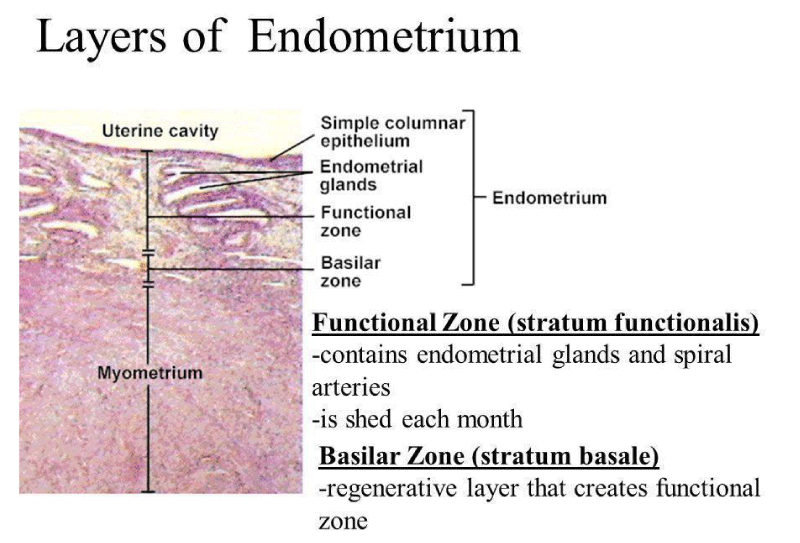
The endometrium is made up mostly of mucosal tissue. It has two layers: The first deep layer, the stratum basalis, Second superficial layer, and the stratum functinalis.
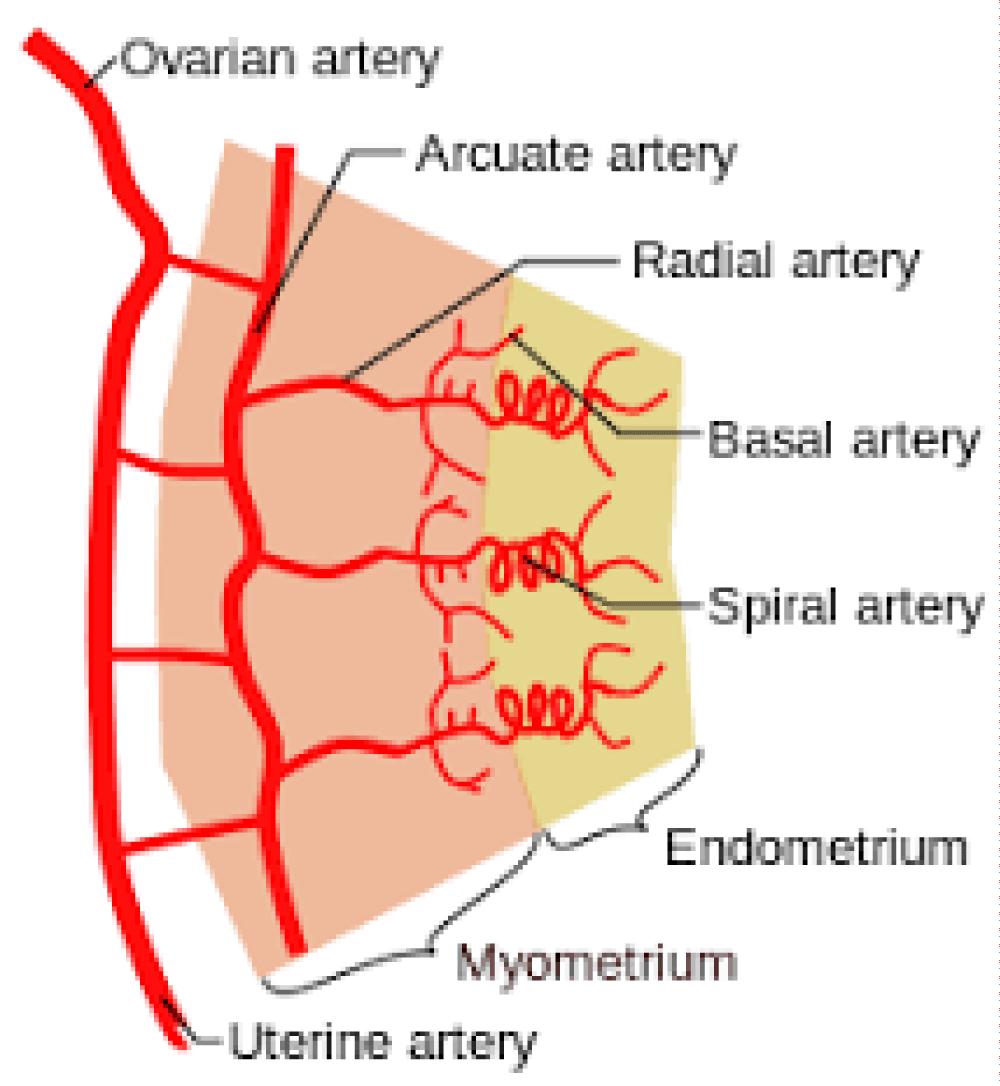
The endometrium consists of a single layer of columnar epithelium plus the stroma on which it rests. The stroma is a layer of connective tissue that varies in thickness according to hormonal influences. In the uterus, simple tubular glands reach from the endometrial surface through to the base of the stroma, which also carries a rich blood supply provided by the spiral arteries. In a woman of reproductive age, two layers of endometrium can be distinguished. These two layers occur only in the endometrium lining the uterine cavity, not in the fallopian tubes.
- The basal layer (stratum basalis), adjacent to the myometrium and below the functional layer, is not shed at any time during the menstrual cycle. The functional layer develops on top of it. The basal layer attaches to the layer of smooth muscle tissue of the uterus called the myometrium. This layer serves as an anchor for the endometrium within the uterus and stays relatively unchanged (Figure 1).
- In the absence of progesterone, the arteries supplying blood to the functional layer constrict, so that cells in that layer become ischemic and die, leading to menstruation.
- The functional layer (stratum functionalis) is adjacent to the uterine cavity. The second layer is dynamic. It changes in response to the monthly flux of hormones that guide the menstrual cycle. It's the part of the endometrium where a fertilized egg (or blastocyst) will implant if conception takes place.
Figure 1: Cross sectional Microscopy image of Layers of Endometrium.
This layer is built up after the end of menstruation during the first part of the previous menstrual cycle. Proliferation is induced by estrogen during the follicular phase of the menstrual cycle, and later changes in this layer are generated by progesterone from the corpus luteum (luteal phase). It is adapted to provide an optimum environment for the implantation and growth of the embryo. This layer is completely shed during menstruation.
The blood supply to the basal portion of the endometrium is via the straight basal arteries, and the spiral arterioles supply the functionalis layer. Both the straight basal arteries and the spiral arterioles arise from the radial arteries at the myometrial‐endometrial junction. The radial arteries arise from the arcuate arteries within the myometrium. The endometrium comprises luminal and glandular epithelia, stromal fibroblasts, and vascular smooth muscle cells.
Physiology of the endometrium
The endometrium is one of the most dynamic organs in the body. Physiologically, the endometrial thickness has a wide range. It can respond to an endocrine environment by an increase in thickness in preparation for implantation or by sloughing its lining in preparation for a new cycle. Its dysfunction or anatomic disturbance can result in menorrhagia, and its muscle can allow expulsion of the effluent and yet preserve the integrity of the contents inside. In response to medication, the endometrium can increase or decrease in thickness at any time during the menstrual cycle and can alter the size of the anatomic pathologies within by an altering its vascularity and proliferation. The response of the endometrium during the menstrual cycle reflects its dynamic nature. Once the dynamic nature of the endometrium is understood, it can be manipulated therapeutically.
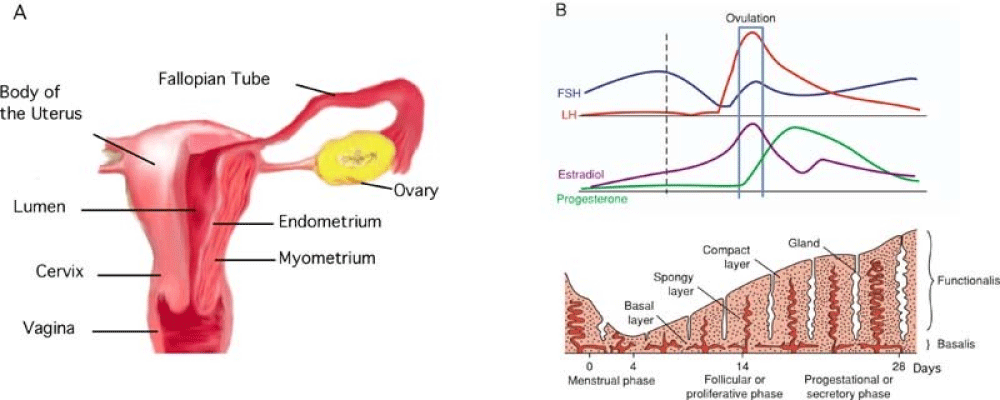
The endometrium can be classified generally into two layers. The functionalis (subdivided further into spongiosa and compacta) sits adjacent to the luminal epithelium, and the basalis sits adjacent to the myometrium. The functionalis undergoes significant change in response to the menstrual cycle as compared to the basalis.
The proliferative phase: The endometrium is less than 2 mm thick in the early proliferative phase. It consists of glands and stroma. The epithelium consists of a single layer of columnar cells. Most of the functional layer is shed with each menstrual period. The basal layer is closest to the uterine muscle and serves as the reservoir for regenerating the other layer. Mitotic activity increases from the start of menses, and by day 5 after the onset of menses, regeneration of the epithelium is complete. Then, in response to estrogen, there is glandular hyperplasia and an increase in the height of the glandular epithelial cells and pseudo stratification as the endometrium prepares for ovulation.
The secretory phase: With ovulation, the endometrium prepares for possible implantation. There is a decrease in mitotic activity in response to progesterone. Progesterone induces decidual changes within the endometrium. The glandular epithelial cells begin to accumulate glycogen-rich vacuoles at their base, and mild secretory activity becomes apparent. Under the influence of humoral and local factors, there appear spiral arteries and the increasing coiling of the arteries and glands. The microscopic morphology of the endometrium changes almost daily and is easily identifiable by the pathologist. The thickness of the endometrium increases to approximately 6 mm. There is a significant amount of stromal edema. Both these features, the thick endometrium and vascular edema, can be recognized by hysteroscopy.
Menstruation: Before menstruation, the stromal network begins to degenerate, with decrease in the secretory products and an infiltration by monocytes and leukocytes. There is actually a decrease in the height of the endometrium from the lack of secretory products and a breakdown in the extracellular matrix. With a decrease in serum estrogen and progesterone from decreased function of the corpus luteum, further constriction and coiling of the spiral arterioles occurs. Prostaglandins are secreted, causing contraction of the uterine smooth muscle, ischemia of the spongiosa and compacta, and finally sloughing of endometrial tissue. Bleeding stops under the increasing influence of estrogen, which causes a prolonged vasoconstriction and fibrin clot formation over denuded endometrial surfaces. An increase in estrogen levels reinitiates the process of regeneration.
The cyclic change of human endometrial cells are controlled by the interaction between hypothalamus, pituitary gland and ovaries, thus making the endometrium proliferate, differentiate, exfoliate and then reproduce. The menstrual cycle is divided into three phases which are called follicle, ovulatory and luteal phase by the morphological change of the ovarium. The endometrial cycle is also classified to proliferative, secretory and menstrual phase. Estradiol (E2) stimulates the proliferation of endometrial cells by the indirect positive mechanism activated by the binding of E2 to E2 receptor. Growth factors (IGF- I, EGF, TGF- alpha etc.) induced by the transcription of the gene promote the proliferation of endometrial cells. Progesterone (P) has antagonistic effects on E2 actions and transform proliferative phase to secretory phase in endometrium. It is suggested that the possible mechanism of carcinogenesis of normal endometrium is the progression of endometrial hyperplasia due to the prolonged and unphysiological exposure to E2. The additional role of oncogenes (fos, fms, myc, myb, erb-B, neu) and growth factors on the mechanism of carcinogenesis of hyperplasia to cancer is very interesting (Figure 2).
Figure 2: (A) Anatomy of uterus; (B) Physiological changes of endometrium.
Endometrium during Embryo transfer
An endometrial thickness of less than 7 mm decreases the pregnancy rate in in vitro fertilization by an odds ratio of approximately 0.4 compared to an EMT of over 7 mm. However, such low thickness rarely occurs, and any routine use of this parameter is regarded as not justified.
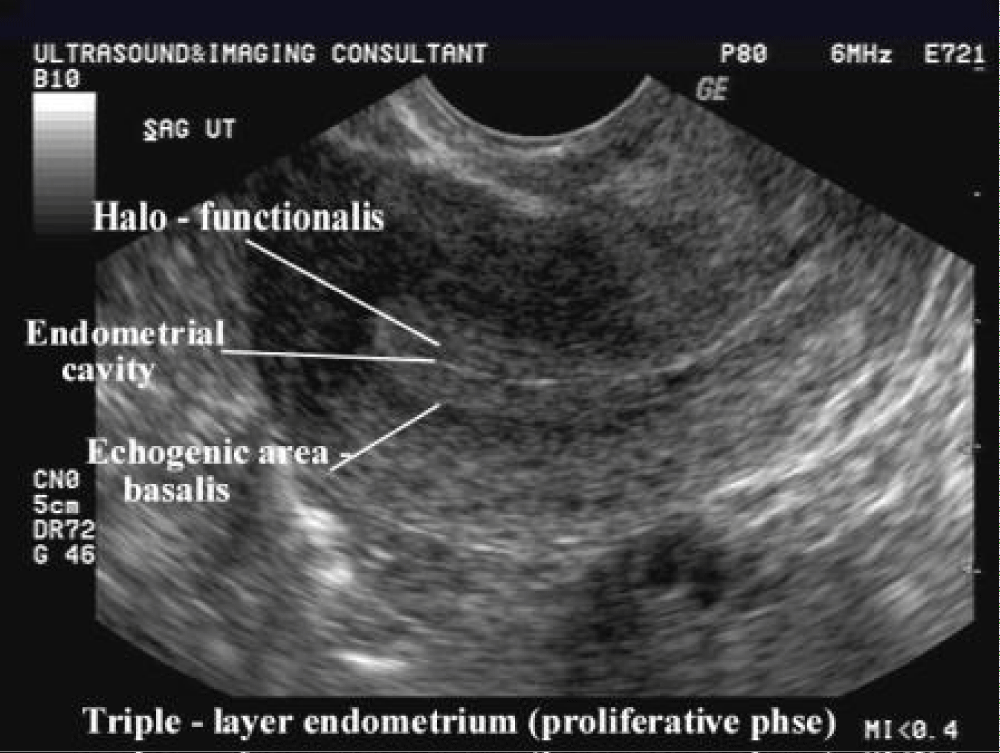
Observation of the endometrium by transvaginal ultrasonography is used when administering fertility medication, such as in vitro fertilization. At the time of embryo transfer, it is favorable to have an endometrium of a thickness of between 7 and 14 mm with a triple-line configuration, which means that the endometrium contains a hyperechoic (usually displayed as light) line in the middle surrounded by two more hypoechoic (darker) lines. A triple-line endometrium reflects the separation of the stratum basalis and functionalis layers, and is also observed in the periovulatory period secondary to rising estradiol levels, and disappears after ovulation (Figure 3).
Figure 3: Ultrasound 2 Dimensional Grey scale measurement of Endometrial thickness.
Main functions of endometrium
- Cyclic alterations of the uterine glands and blood vessels during the course of the menstruation, as preparation for the implantation.
- Location where the blastocyst is normally implanted.
- Location where the placenta develops.
Significance of endometrial thickness
The significance of the endometrial thickness has been investigated in numerous studies and in meta-analyses. The investigations are essentially limited to in vitro fertilization treatments (IVF) with high-dose stimulation therapy and intrauterine insemination treatment (IUI) with different ovarian stimulation regimes.
The importance of the endometrium for the development and maintenance of pregnancy is clearly proven. However, it is unclear which endometrial factors are of relevance (1). Histological examination in couples who wish to conceive makes little sense as a biopsy would be necessary. In transvaginal ultrasound evaluation endometrial thickness, the echo pattern and endometrial perfusion are evaluated (2). Ultrasound analysis of endometrial thickness (EMT) is most commonly performed, as this is the easiest, noninvasive and best reproducible technique.
In IVF treatments, a thin endometrium is associated with lower pregnancy rates. The clinical pregnancy rate is related to a lower chance of pregnancy if endometrial thickness is ≤ 7 mm (OR 0.42, 95% CI: 0.27, 0.67).The data regarding a thick endometrium is not so clear. A previous study described reduced pregnancy rates in women with endometrium > 14 mm, whereas other studies did not find decreased or found even increased pregnancy rates.
Endometrial and receptivity factors are important in the success of in vitro fertilization and embryo transfer (IVF-ET) cycles. Many researches have been done to improve it [7]. It has been shown that endometrial thickness of less than 7 mm has a negative effect on pregnancy rate. Standard in vitro fertilization (IVF) treatments affect less than 1% of women with thin endometrium. It is a crucial problem for both patient and physician. It can lead to unwanted cancellation and delay in treatment. The correlation between endometrial thickness and receptivity has been mentioned in various studies. However, some studies have not reported such a correlation [8]. When endometrium is not appropriately thickened for embryo transfer, the physician uses drugs such as aspirin, sildenafil, pentoxifylline and tocopherol-f. Still, the endometrium remains unresponsive in some cases.
How thin is really thin?
A thin endometrium is mostly defined as an endometrial thickness of < 7 mm on ultrasound although a cut-off value of 6 mm and 8 mm has also been used [7]. Two hormone produced by ovaries thicken and prepare uterine lining for implantation. Estrogen causes thin uterine lining to thick. Progestrone causes thickened uterine lining to develop the characteristics needed for implantation [9-11].
Endometrial changes reflect hormonal changes throughout the menstrual cycle. The functional layer of the endometrium starts growing under the influence of estrogen (E) till it reaches a maximum at the onset of the luteinizing hormone (LH) surge. The pre- and post-ovulatory rise of progesterone (P4) herald's secretory changes in the endometrium and these are paralleled by changes in the endometrial pattern (EnP). On ultrasound endometrial thickness increases in the follicular phase and endometrial character changes from a hypoechoic trilaminar one to a compact hyperechoic look post ovulation. A trilaminar endometrium on the day of ovulation trigger is associated with an increased probability of pregnancy while a hyperechoic character signals failure.
Incidence of thin endometrium
Though controversial endometrial thickness has been used to predict the possibility of pregnancy in ART cycles; a thin endometrium being associated with poor success rates after IVF irrespective of the causative factor. However, Pregnancies have been reported even at endometrial thicknesses as low as 4 mm or 5 mm. suggesting that receptivity may not necessarily be related to endometrial thickness. A thin endometrium is seen more often in older women probably because of decreased vascularity. An incidence of 5% has been reported in women under 40 years and 25% in women over 40 years in natural cycles. Kasius, et al. 2014, reported an incidence of 2.4% in their meta-analysis that included 1170 patients undergoing IVF. Kumbak, et al. 2009, looked at the cycle characteristics and outcomes of 175 patients with an endometrial thickness of < 7 mm on the day of oocyte retrieval. Patients were stratified according to three endometrial thickness groups ≥ 4 mm and < 5 mm, ≥ 5 mm and < 6 mm, and ≥ 6 mm and < 7 mm. Pregnancy rate (PR) and implantation rate (IR) did not show a statistically significant difference among the three groups though an increasing trend in values was observed as endometrial thickness increased (Tables 1,2).
| Table 1: Endometrial Thickness and Ultrasound imaging of foetal pole and cardiac activity (Fischer exact test, p - value = 0.466, non-significant). | |||
| Characteristic | Clinical Pregnancy |
No Pregnancy | Total |
| Endometrium > 6 mm | 28 | 33 | 61 |
| Endometrium < 6 mm | 5 | 3 | 8 |
| Total | 33 | 36 | 69 |
| Table 2: Endometrial thickness and ongoing pregnancy till second trimester. | |||
| Characteristic | Ongoing Pregnancy |
No Pregnancy | Total |
| Endometrium > 6 mm | 22 | 39 | 61 |
| Endometrium < 6 mm | 1 | 7 | 8 |
| Total | 23 | 46 | 69 |
The clinical PR (CPRs) and miscarriage rates (MRs) of the study group were compared with 5573 patients undergoing IVF during the same period who had an endometrial thickness of ≥ 7 mm. The CPR's were 26% and 51% (p < 0.0001), MR 31% and 17% (p = 0.02) in patients with endometrium < 7 mm and > 7 mm, respectively. The CPR, IR, and live birth rate (LVBR), per embryo transfer (ET) of patients with a thin endometrium, were further assessed according to age, number of oocytes retrieved and embryos transferred. Significantly better results were obtained when the patient's age was < 35 years or the number of retrieved oocytes was > 5 or the number of embryos transferred was three or more. The authors suggest that if the endometrium is < 7 mm on the day of oocyte pick-up, the patient should be offered total embryo freezing unless the number of oocytes recovered is > 5, the number of embryos available for transfer ≥ 3. Even though this study supports the view that an endometrium < 7 mm compromises chances of pregnancy, results based on stratification by age, oocyte numbers, and number of embryos transferred indicate that endometrial thickness is not the only determinant of treatment outcome.
A contrary view was given by Kasius, et al. 2014, based on a systemic review and meta-analysis of 1170 studies (22 of which were of moderate quality). Their review suggests that Eth cannot be used as a parameter to decide on cycle cancelation, freezing of all embryos or discontinuing IVF treatment. A thin endometrium (≤ 7 mm) occurred infrequently - 2.4% of the reported cases (260/10 724) and the estimated summary receiver operating characteristic curve indicated a virtually absent discriminatory capacity of Eth in the prediction of pregnancy. However, there was a trend toward lower ongoing pregnancy and LVBRs for these women (odds ratio [OR] 0.38 [95% confidence interval [CI] 0.09 - 1.5]). The probability of clinical pregnancy for Eth ≤ 7 mm was significantly lower compared with that for an Eth > 7 mm (23.3% vs. 48.1%, OR 0.42 [95% CI 0.27 - 0.67]). The positive predictive value for the outcome of clinical pregnancy was 77%, and the negative predictive value was 48%. One can conclude from this review that Eth can give us probability but cannot be predictive of pregnancy.
The adverse effect of controlled ovarian hyper stimulation (COH) on ER is well established, and elective freezing of embryos with the subsequent transfer in a hormone replacement therapy (HRT) cycle is advocated to improve implantation. The effect of endometrial thickness on PR in a frozen embryo transfer (FET) cycles has also been examined [7], found that an endometrial thickness of 9 - 14 mm measured on the day of p supplementation was associated with higher implantation and PRs compared with an endometrial thickness of 7 - 8 mm. Dix and Check, 2010, in a retrospective analysis looked at PR in patients with Eth of < 6 mm. Of the 35 patients, there were only three pregnancies and only two patients delivered. The overall PR was 8.5% per transfer and LVBR 5.7% per transfer PR in FET group was higher being 14.2%. The maximal thickness at which a patient achieved LVB was 5 mm in the FET group. A recent study by the same group compared the LVBR, PR, and IR between fresh and FET (Frozen Embryo Transfer) in endometrium <6 mm. The IR and LVBR at 4 - 5 mm in fresh versus frozen transfer cycles was 10.6% versus 27.2% (p = 0.079) (IR) and 10% versus 36% (p = 0.0325) (LVBR) being higher in the HRT-FET cycles. ET was not attempted when endometrium was < 4 mm. Both the studies from this group imply that a hormone replacement therapy (HRT) cycle could mitigate the negative effects of controlled ovarian hyperstimulation (COH) even in patients with a thin endometrium.
Oocyte donation cycles are ideal to measure the independent effect of endometrial thickness as a parameter of Endometrial Receptivity because there is lower variability of embryo quality [12], studied the effect of Eth on reproductive outcome in oocyte donation cycle using 6 mm and 8.2 mm as the cut-off for a thin endometrium. There were no statistically significant differences in Clinical Pregnancy Rate (29.6% vs. 30.0%) and Live Birth Rate (16.7% vs. 23.6%) in women with endometrium <6 mm compared with Eth > 6 mm, respectively. However, more live births were observed for an endometrial thickness cut-off of 8.2 mm than for thinner endometrium. It is possible that a thin endometrium is unable to support pregnancy development after implantation, resulting in more miscarriages due to intrauterine (IU) fetal deaths. It was also observed that a lower proportion of patients with endometrium thinner than 6 mm exhibited Endometrial Pattern Grade A and a higher proportion exhibited Grade C.
Embryo quality plays a major role in implantation and is one of the determining factors for pregnancy outcome. Gingold, et al. 2015, sought to evaluate the relationship of endometrial thickness to pregnancy outcome after euploid ET. Having transferred only euploid embryos after pre implantation genetic screening they found that Endometrial thickness (≤ 8 vs. > 8 mm), on the day of trigger or ET, had no significant correlation with IR or clinical outcomes across all age groups (23.4 - 44.4 years, mean: 36.1 ± 4.0 years) in either fresh or FETs. However, a completely homogenous Endometrial Profile at trigger did correlate with a low IR. The Endometrial thickness ranged from 4.4 to 17.9 mm (mean: 9.7 ± 2.2 mm) at fresh ET day, and from 4.2 to 17.7 mm (mean: 9.1 ± 2.1 mm) at FET day. The subset of patients with endometrium ≤ 7 mm was too small to analyze for statistical significance. The authors also suggest that these results may not apply to patients whose Endometrial thickness or Endometrial Progesterone is altered because of endometrial pathology (e.g., from Asherman's syndrome, Endometrial tuberculosis, or an autoimmune disorder). Endometrial damage by disease can lead to reduced vascularity and fibrosis.
Factors affecting endometrial thickness
- Permanent damage to basal Endometrium
- Reduced blood flow to endometrium
- Endometrial resistance to circulating Estrogen
- Over exposure to testosterone
Causes of thin endometrium
Thin endometrium can result from various factors the most common being inflammatory and iatrogenic. Poor vascularity and low estradiol values can also lead to poor endometrial growth. The endometrium can also be inherently thin in some women [12].
- Inflammatory causes: Acute or chronic infection can lead to the destruction of the basal layer of the endometrium. In India, genital Koch's is the most common cause of thin endometrium. Since healing takes place by fibrosis, it leads to the destruction of the endometrium and shrinkage of the uterine cavity. Regeneration of endometrium even after complete treatment is very difficult as fibrosis destroys the basal layer.
- Iatrogenic: Surgical causes are repeated or vigorous curettage damages the basal layer of endometrium. Hysteroscopic myomectomy, polypectomy, or laparoscopic myomectomy where the cavity is opened may lead to intrauterine adhesions. Medical causes are indiscriminate use of drugs such as clomiphene citrate.
- Idiopathic: Thin endometrium may not necessarily be secondary to a disease process. It can result from individual uterine architecture [13] or the intrinsic properties of endometrium that affect its growth [14].
Miwa, et al. 2009 [10], demonstrated that thin endometrial were characterized by poor growth of glandular epithelium, high uterine blood flow impedance, decreased Vascular Endothelial Growth Factor (VEGF) expression, and poor vascular development. They postulated that a high blood flow impedance of radial arteries acting as the trigger impaired the growth of the glandular epithelium and resulted in a decrease in VEGF levels in the endometrium. Low VEGF, in turn, causes poor vascular development, which further decreases blood flow in the endometrium. This vicious cycle leads to a thin endometrium.
Other causes:
- low levels of estrogen
- Inadequate blood flow
- Fibroids
- Amenorrhea/Abnormal Periods
- Pelvic Inflammatory Disease (PID)
- D&C or any pelvic surgeries
- Intrauterine Adhesions
- Excessive use of Clomiphene (Clomid)
- Poor quality of endometrial tissue
- Long term use of Birth control Pills.
Endometrial thickness & receptivity
Endometrial thickness is measured by transvaginal ultrasound as the maximal distance between the echogenic interfaces of the myometrium and endometrium in the plane through the central longitudinal axis of the uterine body [15]. An endometrial thickness of < 7 mm at the time of embryo implantation is considered suboptimal in ART.
On ultrasound endometrial thickness increases in the follicular phase and endometrial character changes from a hypoechoic trilaminar one to a compact hyperechoic look post ovulation. A trilaminar endometrium on the day of ovulation trigger is associated with an increased probability of pregnancy while a hyperechoic character predicts failure [16-18].
- Endometrial Pattern and Endometrial Receptivity a triple line appearance or multilayered endometrium on the day of the ovulation trigger is defined as Grade A or receptive.
- A homogenous appearance or non-multilayered endometrium is defined as Grade C or nonreceptive.
- Progesterone secretion initiates changes in the endometrium that are reflected as a homogenous character near the junctional zone and a well-defined central echogenic line. This Endometrial Pattern seen on the day of ovulation trigger is defined as Grade B [19] (Figure 4).
This Endometrial Pattern seen on the day of ovulation trigger is defined as Grade B [19] (Figure 4).
Figure 4: Ultrasound Imaging Pattern of Endometrium.
The ability to predict pregnancy outcome following ET (Embryo transfer) remains elusive and has led to a search for predictive markers. Maternal age, ovarian reserve measurement, and markers of Endometrial Receptivity (ER) have been evaluated in this context. ER is integral to implantation so identification of an accurate marker of implantation would be highly beneficial in assisted reproductive technology (ART) [20]. Despite enormous research in the field of human embryo implantation, the ideal marker of ER remains indefinable. Lack of accuracy, predictive value, and invasive nature of the biochemical and histological markers of ER limit their clinical applicability.
The parameters used to evaluate ER with a traditional two-dimensional ultrasound are an assessment of endometrial thickness and Endometrial Profile. With the advent of three-dimensional and four-dimensional ultrasound, additional factors have been studied to improve the predictive value of this investigative modality. These include measurement of endometrial volume and Doppler sonography of uterine and sub-endometrial blood flow [21,22]. Interestingly, endometrial thickness and pattern still remain the most researched parameters for their predictive value in IVF.
Endometrium pattern, endometrium thickness, and end-diastolic blood flow have been shown to be the most effective combination for evaluation of uterine receptivity.
Endometrial volume
The volume of the endometrium is measured by area tracing from the fundus to the internal cervical os in 10–15 parallel slices in the transverse section after reloading the patient’s data from the disc. In one study endometrial thickness and volume were significantly higher in pregnant (13 mm, 4.5 mL) than in non-pregnant women (10 mm, 3.3 mL; P.01). Patients’ age and the embryo score on day 3 also reported significant differences between patients conceiving and those not conceiving. According to the cutoff level reported previously (2), women were divided into two groups (endometrial volume 2 mL and 2 mL). The pregnancy rate was lower in patients with an endometrial volume 2 mL than in those with a volume 2 mL (10.0% vs. 31.5%; p = not significant" or "p > 0.05) [23]. The authors tried to find a reliable threshold value allowing prediction of the establishment of a pregnancy. The cutoff for endometrial volume giving the best statistical power was 2.5 mL. Pregnancy rate was significantly lower in patients with an endometrial volume < 2.5 mL (PR 9.4%) compared to those with a volume ≥ 2.5 mL (PR 35.0%, p = .004). The negative predictive value for the achievement of a pregnancy was 90.6% at a threshold value of 2.5 mL, with a positive predictive value of 35%. The positive predictive value was low, which was due to the low overall pregnancy rate. When testing 2D measured endometrial thickness as a predictor for embryo implantation results, no cutoff could be found where the pregnancy rates were significantly different. The minimal endometrial thickness associated with a pregnancy was 9 mm [24].
The reproducibility of 3D measurements is high, which is a prerequisite for all analyses (7–11). In this study a threshold value of 2.5 mL for endometrial volume was predictive of failure to establish a pregnancy [25,26], with a negative predictive value of 91%. The positive predictive value, however, was only 35%, due to low overall pregnancy rates. This cutoff value of 2.5 mL has been confirmed by Yaman, et al. They also found a significantly higher pregnancy rate in patients with an endometrial volume 2.5 mL compared to those patients with an endometrial volume of 2.5 mL.
Endometrial volume
Detection rates of endometrial blood flow in the groups of embryo diapause, miscarriage and ectopic pregnancy were significantly lower than that in the intrauterine live foetus group, suggesting an association between the condition of endometrial blood flow [1,2,4] and embryo implantation and development. Our findings also observed that the pregnancy rate and implantation rate of the patients with detected endometrial and sub endometrial blood flow were significantly higher than those with only sub endometrial blood flow detection or those without detected blood flow, while the miscarriage rate in the patients with detected endometrial and sub endometrial blood flow was significantly lower [2,5].
The endometrial thickness and the speed of uterine blood flow had no impact on pregnancy outcomes. These findings support the association between endometrial blood flow and endometrial receptivity [27].
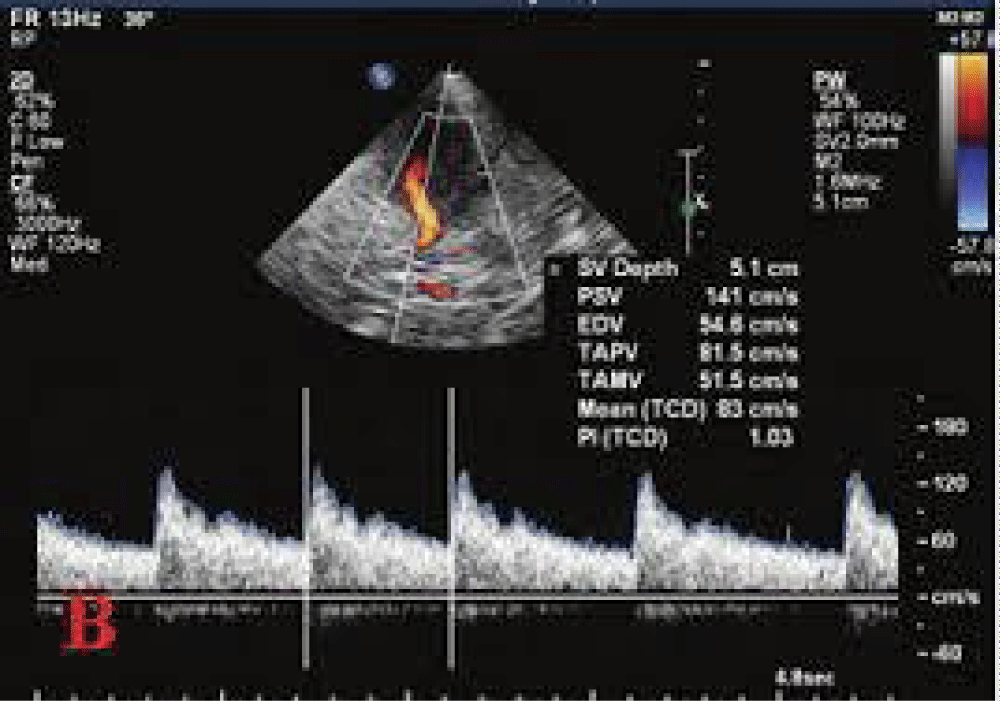
Doppler of uterine artery
Using color Doppler in the two-dimensional mode, flow velocity waveforms were obtained from the ascending main branch of the uterine artery on the right and left side of the cervix before it entered the uterus in a longitudinal plane. Ovarian blood flow were obtained by color Doppler and the average of two ovaries were calculated. Endometrial blood flow was detected by intra-endometrial or the adjacent sub-endometrial regions within 10 mm of the echogenic endometrial borders. Double thickness of the endometrium was measured along the longitudinal axis of the uterus). Thereafter, color Doppler energy was superimposed on the 2-D Doppler studies were performed on selected areas [28,29]. The pulsatility index (PI) and resistance index (RI) of the uterine, ovary and endometrial arteries were calculated electronically when three similar, consecutive waveforms of good quality were obtained. Analysis was used together with computer algorithms to form the endometrial volume and indices of blood flow within the endometrium. The parameters were analyzed by software for: (i) resistance index (RI): the difference between maximal systolic blood flow and minimal diastolic flow divided by the peak systolic flow (S-D/S); (ii) pulsatility index (PI): the difference between maximal systolic blood flow and minimal diastolic flow divided by the mean flow throughout the cycle (S - D/mean); (iii) the ratio between peak systolic flow and lowest diastolic flow (S/D). These three parameters express the resistance to flow from the point of measurement downstream [30].
The patients were divided into three groups according to the condition of the endometrial blood flow in,
- Group A, no endometrial blood flow was detected;
- Group B had sub-endometrial blood flow detected, and
- Group C had both endometrial and sub endometrial blood flow detected.
Early color Doppler ultrasonography is mainly used to assess endometrial receptivity through measuring uterine blood flow. Our study, however, found no association between uterine arterial blood flow and pregnancy outcome, as describe by [31] and we suggest that uterine artery S/D, RI and PI cannot be used independently to predict endometrial receptivity [32-34] (Figure 5).
Figure 5: Uterine artery Doppler study.
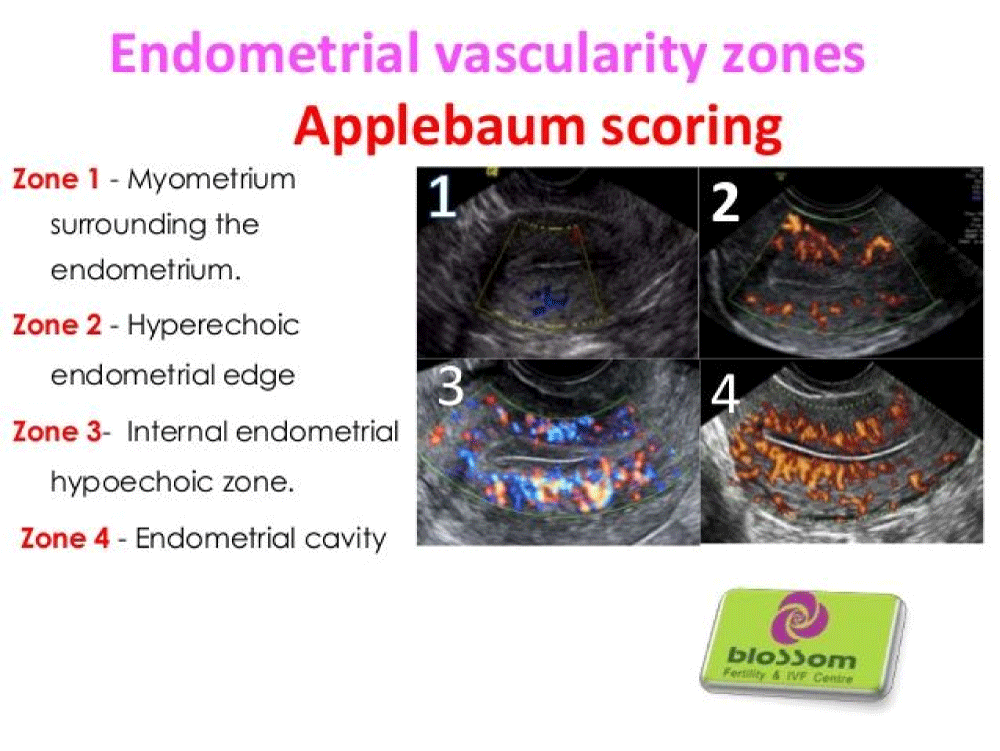
Apple Baum’s vascularity zone
The endometrial and periendometrial areas are divided into the following four zones (Figure 6):
Figure 6: Endometrial Vascularity Zones.
- Zone 1: A 2-mm thick area surrounding the hyperechoic outer layer of the endometrium.
- Zone 2: The hyperechoic outer layer of the endometrium.
- Zone 3: The hypoechoic inner layer of the endometrium.
- Zone 4: The endometrial cavity [26].
Angiogenesis
Angiogenesis plays a key role in different female reproductive developments, including growth of dominant follicles, formation of the corpus luteum, and development of the endometrial pattern. Endometrial angiogenesis is vital for regeneration endometrium after menstruation and to provide a vascularized receptive endometrium for implantation [14,35].The majority of studies has focused on Vascular Endothelial Growth Factor (VEGF) plays a key regulatory role in endometrial angiogenesis, and a number of works stated that VEGF is expressed differentially in the uterine with thin endometrium [36], uterine blood flow serves as an essential marker regulating endometrial growth and is narrowly related to vascular development of the endometrium . Recent improvements in ultrasonography have provided new opportunities for non-invasive evaluation of endometrial perfusion. A significant reduced pregnancy rate in IVF-ET patients with low uterine blood flow, displayed a close relationship between uterine blood flow and uterine receptivity [37,38]. Asherman’s syndrome is a known cause of thin endometrium. Transvaginal ultrasonography usually reveals minimal endometrial thickness in this patients. Impaired uterine perfusion is the reason for atrophy of the remaining endometrium due to restricted exposure to circulating hormones. This syndrome may occur followed myometrial fibrosis that is significantly increased among women with intrauterine adhesions (IUAs) compared to healthy controls [39-42]. (Figure 7).
Figure 7: Angiogenesis.
Implications of impaired angiogenesis
- High impedance of blood flow in radial arteries
- Poor growth of glandular epithelium
- Decreased VEGF expression.
Assessment of endometrial receptivity:
Non-Invasive:
USG – Thickness /Pattern / volume/ blood flow.
MRI
Laser Blood Flowmetry
Invasive:
ERA (Endometrial receptivity assay).
Endometrial preparation
We assessed and recorded pattern and thickness on the day 18 of previous cycle of FET. Method for endometrium preparation is with the exogenous administration of estrogen and progesterone (with or without a gonadotrophin-releasing-hormone (GnRH) agonist. Estrogen administration is started at the beginning of the cycle, causing endometrial development while suppressing dominant follicle development. Estrogen administration is continued until the endometrium reaches a thickness of 8 mm (determined using an ultrasonographic examination), and progesterone is then combined to initiate the secretory changes. This protocol mimics the physiological mid-cycle estrogen–progesterone transition (Figure 1). Estrogen was administered as an oral tablet.
Low-dose aspirin (75 mg once daily) was administered for improving endometrial receptivity. Intramuscular administration of progesterone for luteal support was started from 1 day after ovulation. For hormone replacement treatment cycles, administration of oral estradiol (Progynova; Bayer; Leverkusen) was initial with 2 mg/day from cycle days 1 to 4, 4 mg/day from days 5 to 8, and 6 mg/day from days 9 to 12. Since day 13, transvaginal ultrasound examination (Mindray) was conducted to monitor the endometrial thickness and ovulation and the dose of estradiol was adjusted according to the endometrial thickness. When the endometrial thickness reached 8.0 mm or maximum, 100 mg intramuscular administration of progesterone was provided and maintained for the following 4 days. Thawing and transferring of blastocysts was performed on day 5 after 4 days of progesterone administration.
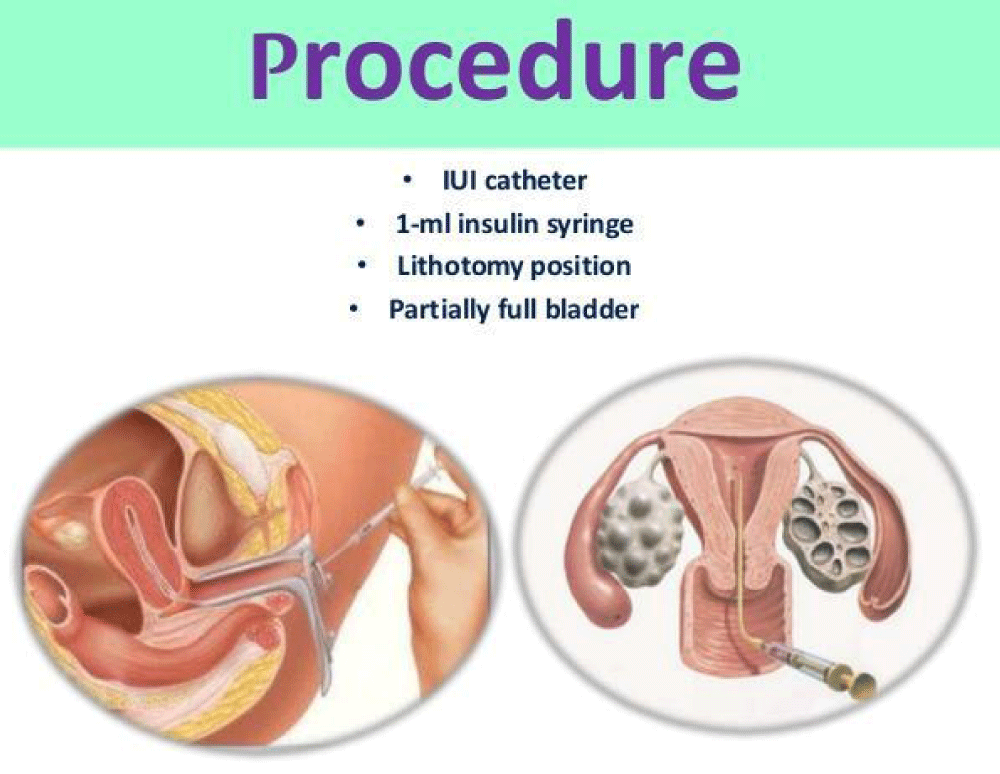
G-CSF (Granulocyte colony stimulating factor): It was started on the day of 9 & also day 12, day 15, that G-CSF may help increase the endometrial thickness and the chance of pregnancy in the patient with extremely thin endometrium. A growth occurred in endometrial thickness and was detected within 48 - 72 hrs of G-CSF administration. IU G-CSF instillation to improve endometrial growth stems from the understanding that the human endometrium contains a small population of mesenchymal stem-like cells that could be responsible for endometrial cyclical growth and reconstruction.
Treatment of thin endometrium: Thin endometrium is identified to adversely affect reproductive success rates after assisted reproductive technology (ART). Several treatment modalities have been presented to patients with thin endometrium, to improve endometrial thickness and the subsequent endometrial receptivity. These approaches comprising hormonal management by estradiol, tamoxifen, human chorionic gonadotropin (hCG) and gonadotropin-releasing hormone (GnRH) agonist, vasoactive agents such as aspirin, vitamin E, pentoxifylline, and sildenafil, intra-uterine infusion of growth factor such as Granulocyte Colony Stimulating Factor (G-CSF) and the latest application of platelet-rich plasma, electrical stimulation, regenerative medicine and presentation of endometrial receptivity array.
Estradiol
Infertile patients, who display inadequate endometrium thickness, will be initially treated with oral estradiol. As the endometrium is a hormone dependent tissue estrogen supports endometrial proliferation by causing spiral artery contraction and decreasing oxygen tension in the functional layer that facilitates embryo implantation [43,44]. The preferred method of estradiol administration is oral, and there are no significant differences between micronized estradiol or estradiol valerate [45]. The prolonged administration of estradiol valerate during controlled ovarian hyperstimulation (COH) cycles for 14–82 days, improve the mean endometrial thickness from 6.7 to 8.6 mm as well as significant increase of pregnancy rate (38.5 vs. 4.3%) [46]. Similarly a case of repeated implantation failure due to unreceptive thin endometrium received extended estrogen supplementation for an extended period, using 16 mg/day of estradiol valerate from the third day of her menstrual cycle for 9 days before COH. IVF in this patient led to a twin pregnancy and delivery at term [47]. In contrast Demir, et al. reported that estradiol supplementation with estradiol hemihydrate 4 mg/day started on the day of hCG among women with thin endometrium undergoing ICSI, did not improve clinical pregnancy rate, implantation rate (16% vs. 10.4%), and endometrial thickness [48]. According to a meta-analysis results, administration of pure ethinyl estradiol (EE) for treatment of thin endometrium, increase the endometrial thickness in comparison to patients whom used placebo. Moreover, the best result were achieved while EE administration is starting on 7th–10th day of menstrual cycle with the dose of 0.02 - 0.05 mg/day for 5 days [49]. As the highest serum and endometrial level of estrogen occur after vaginal administration, it is considered as the desirable route in cases that do not response to the other ways. Therefore, Cetinkaya and colleagues administered estrogen vaginally 25 mg daily from 4th day of the cycle for 15 days in Clomiphene citrate induced cycle. They reported significant increase in endometrial thickness on the day of ovulation in estrogen + clomiphene citrate group compare to the group where only Clomiphene citrate was used, but there was no difference in pregnancy rate [50]. In the same way another study compared oral and vaginal administration of estradiol among donor oocytes recipients. The results demonstrated an increase in endometrial thickness as well as ongoing pregnancy rate when vaginal estradiol administration extended to 4 - 6 weeks in women who failed to achieve acceptable endometrial thickness after oral estradiol administration. Oral estrogens were also used for endometrium preparation in FET, where prior IVF failure was believed to be due to thin endometrium.
Jimenez and colleagues administered oral estradiol 2 mg three times a day from day 1st for 12 days. They stated satisfactory development of endometrium in 67% patients.
Aspirin
The effect of low-dose aspirin on pregnancy rate is still debated in the literatures [51]. Even though some studies reported the positive impact of low dose aspirin on endometrial thickness, pattern, and endometrial blood flow, others claimed that aspirin do not increase success of implantation. Hsieh, et al. found significantly higher percentages of trilaminar endometrium (46.5% vs. 26.2%) and [52,53].
Pregnancy rates (18.4% vs. 9.0%) following low-dose aspirin (100 mg) therapy non-aspirin group. In a systematic review and meta-analysis the effect of low-dose aspirin on likelihood of pregnancy was studied among women undergoing IVF/ ICSI. Clinical pregnancy rate per embryo transfer was similar between patients who received low-dose aspirin and those who used placebo or no treatment (RR 1.09, 95% CI 0.92 - 1.29) [54]. Furthermore, Clinical pregnancy per cycle, spontaneous abortion or ectopic pregnancy per Clinical pregnancy and live birth rate per cycle or embryo transfer were not significantly significant between the mentioned groups. On the basis of a Cochrane study, low-dose aspirin has no substantial positive effect on pregnancy and endometrial thickness [55].
L- arginine: L-arginine may help improve endometrial thickness [24]. Adequate thickness of the endometrium, or uterine lining, is important for supporting a pregnancy. Women with a thin lining tend to have low pregnancy rates. A thin lining is defined as a lining of less than 8 millimeters (mm). A thin lining may be due to reduced blood flow through the uterine arteries. L-arginine is a precursor to nitric oxide, which increases blood flow by increasing the dilation of the arteries. L-arginine treatment has been shown to increase the thickness of the endometrial lining. In 6 of 9 patients with a thin lining who took L-arginine, the endometrial lining increased to greater than 8 mm.
Sildenafil: Sildenafil citrate — an inhibitor of cGMP-specific phosphodiesterase type 5 (PDE5) - prevents the breakdown of cGMP and increases the effect of nitric oxide on vascular smooth muscles. Sildenafil citrate administration cause an improvement in uterine blood flow, and in combination with estrogen, may promote to the estrogen-induced proliferation of the endometrial lining [56,57].
The expression of vascular endothelial growth factor (VEGF) and Tumor suppressor factor (p53) genes are essential for breaking down the endometrial cellular matrix, normalize cell growth, and induce angiogenesis. These process are required for adequate endometrial blood supply and consequent embryo implantation [58]. Sildenafil citrate causes significant increases in p53 activity and stimulated angiogenic reactions with elevated VEGF [59]. It is found previously that vaginal sildenafil citrate is effective for the treatment of women with repeated implantation failure and leads to increase endometrial thickness and attaining pregnancy in women with poor endometrial growth [60]. Takasaki, et al. [24] evaluated the effect of sildenafil on improving radial arteries blood flow and endometrial growth. Sildenafil citrate increased radial artery-resistance index and endometrial thickness in 92% cases [61]. Recently Zinger et al. have reported two infertility patients with Asherman’s syndrome with successful pregnancy after consuming 25 mg vaginal sildenafil citrate, four times a day for 6 - 14 days during the first half of their cycle [62]. To evaluate the effects of sildenafil administration on the thickness of the endometrium and the implantation rate, Dehghani-Firouzabadi and coworkers conducted a randomized controlled trial. 80 patients who had a history of poor endometrial response and frozen embryos included the study. 40 patients were given sildenafil citrate tablets (50 mg) daily in addition to the estradiol and compared to 40 women who received estradiol only. They concluded that the oral usage of sildenafil citrate is a good approach to improve the endometrial receptivity [63]. Likewise, a recent study examined the effect of adding sildenafil vaginal gel to CC in infertile women with thin endometrium and previous CC failure (5 cycles). In the patients' 6th cycle (CC only), women sustained on CC 100 mg/day for 5 days. In the 7th cycle, women administered usual dose of CC supplemented in conjunction with sildenafil vaginal gel (5 gm, containing 50 mg sildenafil) twice daily from day 8 to day of HCG administration. The finding indicated that sildenafil vaginal gel considerably increased endometrial thickness and uterine blood flow, and may improve pregnancy rate.
Vitamin E and pentoxifylline
The combination of pentoxifylline (PTX) as a derivative of methylxanthine that induce vasodilatation and vitamin E as an antioxidant was used for treatment of thin endometrium. This combination was given to oocyte recipients with inadequate endometrial thickness after vaginal E2 administration. This method results in significantly improved endometrial thickness, pregnancy and delivery rates[64]. In the same way, Acharya, et al. found an improvement in endometrial thickness and pregnancy rate of 40% in treated patients [65] Similarly, it is reported that use of vitamin E cause adequate ET in 52% of patients as well as growth of the glandular epithelium, number of blood vessels and VEGF protein expression. Also, Cicek and colleagues [66] found that Vitamin E administration could improve the endometrial thickness but not implantation and ongoing pregnancy rate in women with unexplained infertility [67]. Pentoxifylline has been used clinically in the treatment of vascular abnormalities, and is also a good choice for radiation-induced fibrosis. However the precise mechanism has not yet been known, the influence is improved while its use in combination with vitamin E [68,69]. One study assessed the antifibrotic effect of PTX by a combination of PTX and tocopherol (vitamin E) in patients with a thin endometrium who were joined an oocyte donation program. Eighteen oocyte recipients who failed to improve endometrial thickness after receiving vaginal micronized estradiol were included the study. The patients received a combination of PTX (800 mg/day) and vitamin E (1000 IU/day) for 6 months. Pregnancy rate, endometrial thickness and ovarian function have been developed.
Tamoxifen: Tamoxifen blocks the actions of estrogen and has a key role in endocrine therapy for the treatment of estrogen receptor positive breast cancer. Wang, et al. compared reproductive outcome in ovarian stimulation cycles using tamoxifen (40 mg/day from day 3 of the menstrual cycle for 7 days) and clomiphene cycles (100 mg/day for 5 days) in patients undergoing intrauterine insemination. It was found that tamoxifen-treated patients have a significantly increased endometrial thickness and pregnancy rate [70]. Similarly, another study reported that switching CC to tamoxifen for ovulation induction in women with adequate follicular recruitment and thin endometrium improves endometrial thickness [71]. Evaluate the effect of tamoxifen on endometrial thickness among patients with thin endometrium undergoing frozen–thawed embryo transfer cycles. 226 women who had endometrial thickness less than 7.5 mm in previous cycles were recruited. Tamoxifen administration improves endometrial thickness in patients after natural cycle as the same as hormone replacement treatment and ovulation induction cycles during FET. However, Patients with PCOS got the most advantage from tamoxifen and achieve higher pregnancy outcomes [71].
Human chorionic gonadotropin (hCG): hCG play a local paracrine role in the endometrium differentiation and endometrial receptivity by regulating different cytokines and growth factors [72-74]. Papanikolaou and colleagues recruited seventeen infertile patients with the history of implantation failures and resistant thin endometrium.
On day 8 or 9 of the estrogen administration (8 mg daily), hCG injection was started subcutaneously by 150 IU daily for 7 days. After a week on hCG priming, on the day 14 or 15 of cycle, the mean endometrial thickness was improved by 0.8 mm. Such as 35.3% of the patients had more than 20% development in their endometrial thickness after hCG priming and 17% achieved an endometrial thickness more than 7 mm [74]. Likewise in a nonrandomized clinical trial the effect of adding hCG to frozen thawed embryo transfer cycles with history of thin endometrium was examined in 28 patients who had two previous failed cycles because of thin endometrium. 150 IU hCG, was administrated intramuscularly from the day 8 of cycle until endometrial thickness reached at least 7 mm. The study suggested that adding hCG to the conventional method is effective and improved endometrial thickness and pregnancy outcomes significantly [75,76].
Granulocyte colony stimulating factor (G-CSF): It is stated that G-CSF may increase the endometrial thickness and the chance of pregnancy in the patient with extremely thin endometrium [77]. In the same way, Tehraninejad and coworkers [78] reported that infusion of G-CSF in endometrial cavity is safe and possibly effective to increase endometrial growth in patient with thin and unresponsive endometrium [79]. Endometrial perfusion with G-CSF is effective in expanding unresponsive thin endometrium to at least minimal thickness of 7 mm, which was resistant to estrogen and vasodilators. A growth occurred in endometrial thickness can be detected within 48 - 72 h of G-CSF administration [80]. This approach in infertile women on the day of hCG administration and in combination with second fusion resulted in low but very reasonable clinical pregnancy rate [78]. In contrast, a randomized clinical trial in normal IVF patients, G-CSF did not increase endometrial thickness, implantation rates, or clinical pregnancy rates [81]. Some other studies confirmed these results. Eftekhar, et al. failed to demonstrate that G-CSF improve endometrial thickness but they reported G-CSF positive effect on chemical and clinical pregnancy rate of the infertile women with thin endometrium in frozen-thawed embryo transfer cycle [82,83]. Otherwise, Li and colleagues stated effectiveness of G-CSF in developing embryo implantation and clinical pregnancy rate among infertile women with thin endometrium [84] (Figure 8).
Figure 8: Intrauterine G-CSF Procedure.
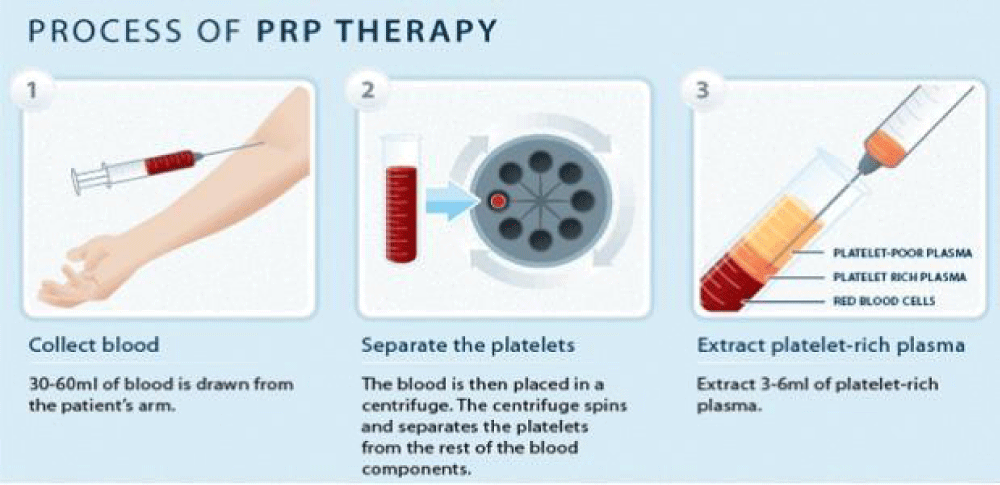
Platelet-rich plasma (PRP): PRP is blood plasma derived from fresh whole blood, enriched with platelets. It is collected from peripheral veins and contains several growth factors and cytokines [85,86]. Upon activation of platelets in PRP, cytokines and growth factors become active and are secreted within 10 min. Recently, PRP has been commonly applied in different clinical situations; however, little is identified about the application of PRP in the treatment of thin endometrium. For the first time, Chang and coworkers introduced intrauterine infusion of PRP as a new approach for the treatment of thin endometrium. They evaluated the efficacy of PRP in the therapy of infertile women with thin endometrium. Five women with thin endometrium after standard hormone replacement therapy (HRT) who were candidate for IVF included the study. PRP was prepared from autologous blood by centrifugation, and infusion of 0.5- 1 ml of PRP into the uterine cavity on the 10th day of HRT cycle was performed [87,88]. If endometrial thickness failed to improve 72 h later, PRP infusion was done 1–2 times in each cycle. Embryos were transferred while the endometrium thickness reached more than 7 mm. endometrial growth as well as successful pregnancy occurred in all the patients after PRP infusion [89]. In the same way, ten patients with the history of inadequate endometrial growth in FET cycles were enrolled in another study. Intrauterine infusion of PRP was performed for all patients. According to the results endometrial thickness increased and 50% of patients achieved pregnancy [90,91] (Figure 9).
Figure 9: Platelet-rich plasma Therapy.
Stem cell therapy
It is indicated that endometrial stem cells are present in both basalis and functionalis layers of the human endometrium, and these cells play a key role in regenerating the endometrial lining during each menstrual cycle [92]. It is also found that human endometrial adult stem cells are capable to produce human endometrium after transplantation in NOD-SCID mice renal capsules Z. Jing, et al. [93], other studies claimed that transplanted BMSCs into rats' uterine cavity [84] as well as transplantation by tail vein IV injection [94] resulted in a significantly thicker endometrial lining and higher expression of cytokeratin, vimentin, integrin αγβ3, and leukemia inhibitor factor. Likewise it is revealed by Taylor and colleagues that non-endometrial stem cells such as hematopoietic and non-hematopoietic bone marrow-derived stem cells (BMDSCs) could induce endometrial regeneration [95]. Interestingly male origin BMDSCs were found in endometrium of female bone marrow transplantation recipients [96]. Moreover, it is demonstrated that BMDSCs induce regeneration of endometrial cells via paracrine signaling [97,98] and this effect is twofold higher during uterine injury rather than monthly cyclic regeneration of the endometrium [99]. Furthermore the influence of BMDSC on endometrial renewal after injury was confirmed in several animal studies [100,101]. On the other hand Hunter, et al. did not found any significant improvements in endometrial lining following stromal vascular fraction cells (SVF) treatment [102].
Based on aforementioned researches, the effects of bone marrow stem cells in the treatment of Asherman’s syndrome and thin endometrium were evaluated in murine model. The numbers of conceived mice were 9 of the 10 in the BMDSC transplant group in comparison with 3 of 10 in the non-transplanted group [103]. Similarly, Kilic and coworkers treated Asherman’s syndrome among Wistar albino rats using either mesenchymal stem cells (MSCs) or oral estrogen or combination of these two methods. Fibrosis decreased but vascularization and immune histochemical staining increased in all treated groups compare to controls [104]. In a recent study, seven types of BMDCs in the endometrial renewal in mice were investigated and results reported that MSCs and EPCs together with mOct4(+) BM-HypoSCs prompted the highest degree of regeneration . There are few reports regarding human application of stem cells for the treatment of thin endometrium. In a plan of autologous stem cells therapy, endometrial angiogenic stem cells were isolated from bone marrow of a patient with Asherman's syndrome. These cells were infused into the endometrial cavity and the results were endometrial thickness of 8 mm and a confirmed clinical pregnancy following IVF [105]. Similarly six cases of refractory Asherman's syndrome with the history of failed standard treatment received subendometrial autologous stem cell implantation followed by exogenous oral estrogen therapy. The endometrial thickness was significantly improved in comparison with pretreatment condition. Additionally five women of six patients resumed menstruation [106]. In a most recent experimental study, 16 patients with refractory Asherman's syndrome or endometrial atrophy received autologous CD133 + BMDSCs through the spiral arterioles by catheterization. Endometrial thickness increased about 2.4 mm and 1.5 mm in Asherman's syndrome and endometrial atrophy patients respectively. Three patients obtained spontaneous pregnancy as well as seven pregnancies which attained after ART [107].
Endometrial receptivity array (ERA)
Challenges to catch an appropriate marker for endometrial receptivity led to definition of a molecular test; the endometrial receptivity array (ERA). It is a molecular diagnostic tool based on the transcriptomic signature of human endometrial receptivity. The test comprises 238 genes which are differentially expressed between varied genetic profiles. This array is able to predict personalized window of implantation irrespective of her endometrial histology. The bioinformatics predictor categorizes an endometrial sample as “receptive” or “non-receptive” [108]. Sensitivity and specificity of ERA are 0.99 and 0.88 respectively [109]. Cruz and Bellver reported a healthy term live birth in a 35-year-old cancerous woman following treatment with chemotherapy and radiotherapy. The woman suffers ovarian failure, hypoplastic uterus and atrophic endometrium. The recognition of a receptive endometrium using ERA and ovum donation resulted in a successful pregnancy in this poor prognosis case [110-121]. Another study used personalized embryo transfer on the day elected by ERA in the Indian population. After personalized ET in patients with persistently thin endometrium, the pregnancy rate of 66.7% was attained in this group. Application of ERA revealed that the endometrium in 75% of these patients was receptive regardless of its thickness of 6 mm or less.
In conclusion, a receptive endometrium is an essential part of embryo implantation process, and inadequate endometrial growth is associated with lower possibility of pregnancy. Many treatment modalities have been applied to improve endometrial receptivity, but their efficacy remains controversial. Large randomized trials should be conducted to evaluate method, dosage, and timing of administration and outcomes of these treatment approaches. Stem cell therapy is considered as a very favorable option for the most cases, even though, more investigation is necessary to evaluate the safety and cost-effectiveness of this technique. ERA is an accurate test for diagnosing endometrial receptivity and could establish a personalized window of implantation in cases with thin endometrium.
Complication of thin endometrium
Patients who conceive in the setting of a thin endometrium have a significantly increased risk of early pregnancy loss, namely miscarriage and ectopic pregnancy. These patients also have a two fold increase in low birth weight and preterm delivery, as well as a significantly higher risk of intrauterine growth restriction and composite adverse perinatal outcomes.
In addition to the lower probability of conception, a thin endometrium in assisted reproductive technologies appears to be associated with both early and late pregnancy complications. These pregnancies thus warrant special attention and close follow-up from obstetricians.
- Miscarriage
- Ectopic pregnancy
- Low birth weight & preterm delivery.
Prevention of thin endometrim
The modified Clomiphene Citrate (CC) treatments provide an alternative to proceeding to gonadotropin therapy for patients with a thin endometrium as a result of standard CC treatment. The present study provides important information to prevent thin endometrium in patients undergoing CC treatment. However, our ultimate goal of CC treatment for infertile patients is a successful pregnancy and live birth. Large scale-RCT will be necessary to evaluate the efficacy of the modified CC treatment on successful pregnancy in patients with a history of a thin endometrium caused by the standard CC regimen.
The thin nonresponsive endometrium of the Asherman's case described in this issue was successfully treated with intrauterine transplantation of bone marrow fibroblasts and MSC and concurrent curette, both of which may have independently or possibly synergistically promoted the growth of endometrial cells. The bone marrow cells by production of trophic factors promoting angiogenesis and tissue growth and the curette by stimulating dormant endometrial stem/progenitor cells into active cell cycle to regenerate endometrial tissue.
Further studies are also required to delineate the mechanisms responsible for these successful treatments of Asherman's syndrome, a previously intractable infertility disorder.
Study population
A total of 69 patients diagnosed as thin endometrium were included in this retrospective study. The diagnosis of thin endometrium by ultrasound guidance.
Inclusion criteria
- Age group 25-45,
- Two or more blastocyst (grade3/4) available for replacement
- Normal uterine cavity in hysteroscopy.
Exclusion criteria
- Presence of endometrial polyp
- Uterine Anomalies
- Adenomyosis
- Hydrosalphinx
- Previous history of D & C
- Previous history Endometritis.
Monitoring
- The endometrium measured transvaginaly in the sagittal plane at the thickest portion near the fundus ON DAY 2, DAY 9, DAY12 AND DAY15 OF FROZEN EMBRYO TRANSFER CYCLE.
- THAWING SCAN is done trans abdominally 2 days before embryo transfer
- EMBRYO TRANSFER : IS DONE ON DAY 19
- Luteal support: All the patients received the same luteal support by progesterone since the day of embryo transfer in which progesterone 20 mg was given daily from the day of ET
A total of 69 cases were enrolled in the study. The overall clinical pregnancy rate was 23% and the early miscarriage rate (pregnancy ending before 12 weeks) was 9% and the implantation failure was 46%.
Ninety percent of the study population had primary infertility and 10% had secondary infertility. Seventy-seven patients had ICSI for the first time and 23 patients had previous trials for ICSI.
The endometrial thickness of the study population ranged from 4 to 8 mm. There was no statistically significant difference among groups as regards age, duration of infertility, body mass index (BMI), and base line hormones (FSH, LH, E2 and prolactin level, also serum progesterone on the day FET).
Clinical pregnancy rate increased from 43.75% among patients with an endometrial thickness ⩽7.0 mm to 73.91% among patients with an endometrial thickness of 8.5 mm, with a non-statistically significant difference among the group.
Clinical pregnancy rate was highest in the trilaminar pattern group (69%) followed by the intermediate pattern group (50%) then the echogenic one (38.4%), however, the difference was not statistically significant.
We tried to measure the effect of combined endometrial thickness and pattern on clinical pregnancy rate, so we calculated clinical pregnancy rate according to the endometrial pattern in each endometrial thickness group. In the 4 - 6.9 mm endometrial thickness group, no clinical pregnancy was detected. In the 7 - 8.5 mm, 9 mm endometrial thickness groups, most of pregnant cases were in the trilaminar pattern group. The trilaminar endometrium in the 8.5 - 9 mm thickness group demonstrated the highest clinical pregnancy rate (56.5%) compared to the other groups, with a statistically significant difference.
Statistical analysis- Descriptive and inferential statistics were used to analyze the data. The mean age and standard deviation of study population was is 33.26 + 5.95. Odd's ratio was used to analyze categorical variables. Predictive values of Endometrium > 6 mm was calculated for Biochemical pregnancy, Clinical pregnancy and ongoing pregnancy. Statistical Analysis was done using MEDCALC (Belgium).
- Sensitivity: probability that a test result will be positive when the disease is present (true positive rate). = a / (a+b)
- Specificity: probability that a test result will be negative when the disease is not present (true negative rate). = d / (c+d)
- Positive likelihood ratio: ratio between the probability of a positive test result given the presence of the disease and the probability of a positive test result given the absence of the disease, i.e. = True positive rate / False positive rate = Sensitivity / (1-Specificity)
- Negative likelihood ratio: ratio between the probability of a negative test result given the presence of the disease and the probability of a negative test result given the absence of the disease, i.e. = False negative rate / True negative rate = (1-Sensitivity) / Specificity
- Positive predictive value: probability that the disease is present when the test is positive.
- Negative predictive value: probability that the disease is not present when the test is negative.
- Accuracy: overall probability that a patient is correctly classified.
Sensitivity, specificity, disease prevalence, positive and negative predictive value as well as accuracy are expressed as percentages. Confidence intervals for sensitivity, specificity and accuracy are "exact" Clopper -Pearson confidence intervals.
Confidence intervals for the likelihood ratios are calculated using the "Log method.”
Confidence intervals for the predictive values are the standard log it confidence intervals.
Statistical analysis (Tables 3, 4)
| Table 3: Predictive Value of Endometrial Thickness>6mm for Clinical and Ongoing Pregnancy in Frozen Embryo Transfer Cycles (N = 69). | ||
| Statistic | Value | 95% Confidence Interval |
| Sensitivity | 84.85% | 68.10% to 94.89% |
| Specificity | 8.33% | 1.75% to 22.47% |
| Positive Likelihood Ratio | 0.93 | 0.78 to 1.10 |
| Negative Likelihood Ratio | 1.82 | 0.47 to 7.02 |
| Disease Prevalence (*) | 47.83% | 35.65% to 60.20% |
| Positive Predictive Value (*) | 45.90% | 41.61% to 50.26% |
| Negative Predictive Value (*) | 37.50% | 13.45% to 69.85% |
| Accuracy (*) | 44.93% | 32.92% to 57.38% |
| Table 4: (Statistical Analysis Results) | ||||||
| Test Statistic (95% CI) |
Sensitivity | Specificity | PPV | NPV | LR+ | LR- |
| Clinical Pregnancy |
84.85(68.1 0-94.89) |
8.33(1.75- 22.47) |
45.90(4 1.61- 50.26) |
37.50(13.4 5-69.85) |
0.93(0.78 -1.10) |
1.82(0.47 -7.02) |
| Ongoing Pregnancy |
95.65(78.0 5-99.89) |
15.22(6.34- 28.87) |
36.07(3 2.68- 39.60) |
87.50(47.7 8-98.17) |
1.13(0.97 -1.31) |
0.29(0.04 -2.19) |
Results
| Ratio | 0.5091 |
| 95% CI: | 0.1116 to 2.3216 |
| z statistic | 0.872 |
| Significance level | p = 0.3832 |
Computational notes: The odds ratio (OR), its standard error and 95% confidence interval are calculated according to Altman, 1991.
Thin endometrium to be associated with a lower implantation rate, but no absolute cut off for endometrial thickness exists; good pregnancy rates have been reported in cycles with endometrium < 6 mm, and a successful pregnancy has been reported with endometrial thickness of only 4 mm. Noyes N et al found that clinical pregnancy rate and live birth rate were significantly lower when endometrial thickness was less than 8 mm than when endometrial thickness was ≥9 mm. In the present study, the thinnest endometrial lining for successful clinical pregnancy was 4.8 mm. The implantation rate and ongoing pregnancy in thin endometrium was lower than that in thick endometrium. The relatively lower pregnancy rate observed in this group suggests that more attention needs to be given for endometrial preparation in subsequent cycles of embryo transfers to such patients.
When the thickness measured by ultrasound is < 6 mm, the functional layer is thin or absent, and the implanting embryo would be much closer to the spiral arteries and the higher vascularity and oxygen concentrations of the basal endometrium. The high oxygen concentrations near the basal layer could be detrimental compared with the usual low oxygen tension of the surface endometrium.
Endometrial receptivity can be induced by exogenous administration of estradiol and progesterone in various protocols for endometrial preparation. Estradiol priming contributes to endometrial proliferation and induction of progesterone receptors. Then, the action of subsequent progesterone turns the estrogen-primed endometrium into a secretory structure for embryo survival and implantation. Therefore, for those patients with thinner endometrium in fresh cycles, it would be useful to prolong the duration of estradiol stimulation for optimal endometrial receptivity.
There is a dynamic change of endometrial thickness after progesterone administration in FET (Frozen Embryo Transfer) cycles. After endometrium preparation, the endometrial thickness on day of embryo transfer increased or kept being stable compared with that on day of progesterone initiation in most patients. In addition, an increased endometrium after progesterone administration was associated with better pregnancy outcome. Interestingly, clinical pregnancy outcomes and the increasing rate of endometrium were positively correlated.
For those patients with thin endometrium in ET cycles, additional estradiol stimulation might be helpful for adequate endometrial development. The reasons for low implantation could be a high impedance blood flow of the radial arteries leading to poor endometrial glandular growth in thin endometrium. Poor angiogenesis subsequent to decreased VEGF secretion can also affect endometrial vascularity. This in turn increases chances of poor placentation and inappropriate vascularization leading to early abortions even if pregnancy is established. Transfer of embryos to an endometrium prepared by HRT seems to yield better results than fresh ET. ERA may be applied in such patients to ensure that the embryo is transferred to a receptive endometrium.
Other agents that are used to improve endometrial thickness and vascularity are Vitamin E, L arginine and sildenfil citrate.
In conclusion, our study suggests that endometrial thickness is a good predictor of endometrial receptivity in subsequent FET cycles. In patients with thin endometrium, additional estradiol stimulation might be helpful for adequate endometrial development. Clinical pregnancy and live birth rates decrease for each millimeter of endometrial thickness below 7 mm for frozen–thaw IVF cycles. Nevertheless, viable pregnancy rates remain reasonably acceptable in patients with an endometrial thickness between 4 and 6 mm.
In our center, the endometrial thickness of more than 8 mm has long been used as the marker of adequate endometrial preparation in FET cycles. However, the actual optimal endometrial thickness might be variable among individuals.. However, the mechanism explaining the association between thin endometrium and embryo transfer outcomes is still not clear. It has been speculated that the increasing basal layer endometrium oxygen concentrations in females with thin endometrium might be detrimental for embryo implantation.
However, it is generally accepted that an endometrial thickness below a minimum value of 6 to 8 mm demonstrated negative predictive value for IVF outcomes, and clinical pregnancy as well as live birth rates are significantly higher in patients with an endometrial thickness >9 to 10 mm. Still, implantation can sometimes occur despite a thin endometrium.
It is possible that a lower endometrial thickness may reflect a higher proportion of poor responders or patients with a poorer prognosis for pregnancy due to other factors.
- Jaffe R, Warsof SL. Transvaginal color Doppler imaging in the assessment of uteroplacental blood flow in the normal first-trimester pregnancy. Am J Obstet Gynecol. 1992;164:781–785. Available from: https://doi.org/10.1016/0002-9378(91)90515-s
- Chien LW, Au HK, Chen PL, Tzeng CR. Assessment of uterine receptivity by the endometrial-subendometrial blood flow distribution pattern in women undergoing in vitro fertilization embryo transfer. Fertil Steril. 2002;78:245–251. Available from: https://doi.org/10.1016/s0015-0282(02)03223-5
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. Available from: https://doi.org/10.1016/s0002-9378(16)33500-1
- Dickey RP, Olar TT, Taylor SN, Curole DN, Rye PH, Matulich EM. Relationship of endometrial thickness and pattern to fecundity in ovulation induction cycles: Effect of clomiphene citrate alone and with human menopausal gonadotropin. Fertil Steril. 1993;59:756–760. Available from: https://doi.org/10.1016/s0015-0282(16)55855-5
- Schild RL, Knobloch C, Dorn C, Fimmers R, van der Ven H, Hansmann M. Endometrial receptivity in vitro fertilization program assessed by spiral artery blood flow, endometrial thickness, endometrial volume and uterine artery blood flow. Fertil Steril. 2001;7:361–366. Available from: https://doi.org/10.1016/s0015-0282(00)01695-2
- Dominguez F, Remohi J, Pellecer A, Simón C. Human endometrial receptivity: a genomic approach. Reprod Biomed Oneline. 2003;6:332–338. Available from: https://doi.org/10.1016/s1472-6483(10)61853-6
- El-Toukhy T, Coomarasamy A, Khairy M, Sunkara K, Seed P, Khalaf Y, et al. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril. 2008;89(4):832-839. Available from: https://doi.org/10.1016/j.fertnstert.2007.04.031
- Glissant A, de Mouzon J, Frydman R. Ultrasound study of the endometrium during in vitro fertilization cycles. Fertil Steril. 1985;44(6):786-790. Available from: https://doi.org/10.1016/s0015-0282(16)49038-2
- Mahajan N, Sharma S. The endometrium in assisted reproductive technology: how thin is thin? J Hum Reprod Sci. 2016;9(1):3-8. Available from: https://doi.org/10.4103/0974-1208.178632
- Miwa I, Tamura H, Takasaki A, Yamagata Y, Shimamura K, Sugino N. Pathophysiologic features of “thin” endometrium. Fertil Steril. 2009;91(4):998-1004. Available from: https://doi.org/10.1016/j.fertnstert.2008.01.029
- Senturk C, Erel T. Thin endometrium in assisted reproductive technology. Curr Opin Obstet Gynecol. 2008;20(3):221-228. Available from: https://doi.org/10.1097/gco.0b013e328302143c
- Dain L, Bider D, Levron J, Zinchenko V, Westler S, Dirnfeld M. Thin endometrium in donor oocyte recipients: Enigma or obstacle for implantation? Fertil Steril. 2013;100:1289–95. Available from: https://doi.org/10.1016/j.fertnstert.2013.07.1966
- Strohmer H, Obruca A, Radner KM, Feichtinger W. Relationship of the individual uterine size and the endometrial thickness in stimulated cycles. Fertil Steril. 1994;61:972–5. Available from: https://doi.org/10.1016/s0015-0282(16)56716-8
- Scioscia M, Lamanna G, Lorusso F, Serrati G, Selvaggi LE, Depalo R. Characterization of endometrial growth in proliferative and early luteal phase in IVF cycles. Reprod Biomed Online. 2009;18(1):73-78. Available from: https://doi.org/10.1016/s1472-6483(10)60427-0
- Gonen Y, Casper RF, Jacobson W, Blankier J. Endometrial thickness and growth during ovarian stimulation: a possible predictor of implantation in in vitro fertilization. Fertil Steril. 1989;52(3):446-450. Available from: https://doi.org/10.1016/s0015-0282(16)60916-0
- Barker MA, Boehnlein LM, Kovacs P, Lindheim SR. Follicular and luteal phase endometrial thickness and echogenic pattern and pregnancy outcome in oocyte donation cycles. J Assist Reprod Genet. 2009;26:243–9. Available from: https://doi.org/10.1007/s10815-009-9312-z
- Mercé LT, Barco MJ, Bau S, Troyano J. Are endometrial parameters by three-dimensional ultrasound and power Doppler angiography related to in vitro fertilization/embryo transfer outcome? Fertil Steril. 2008;89:111–117. Available from: https://doi.org/10.1016/j.fertnstert.2007.02.029
- De Geyter C, Schmitter M, De Geyter M, Nieschlag E, Holzgreve W, Schneider HP. Prospective evaluation of the ultrasound appearance of the endometrium in a cohort of 1,186 infertile women. Fertil Steril. 2000;73(1):106-113. Available from: https://doi.org/10.1016/s0015-0282(99)00484-7
- Friedler S, Schenker JG, Herman A, Lewin A. The role of ultrasonography in the evaluation of endometrial receptivity following assisted reproductive treatments: a critical review. Hum Reprod Update. 1996;2(4):323-335. Available from: https://doi.org/10.1093/humupd/2.4.323
- Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. 2020 Oct 28;10(10):CD006359. Available from: https://doi.org/10.1002/14651858.cd006359.pub3
- Grunfeld L, Walker B, Bergh PA, Sandler B, Hofmann G, Navot D. High-resolution endovaginal ultrasonography of the endometrium: A noninvasive test for endometrial adequacy. Obstet Gynecol. 1991;78:200–4. Available from: https://pubmed.ncbi.nlm.nih.gov/2067763/
- Bonilla-Musoles F, Raga F, Osborne NG, Castillo JC, Bonilla F Jr. Endometrial receptivity: Evaluation with ultrasound. Ultrasound Q. 2013;29:3–20. Available from: https://doi.org/10.1097/ruq.0b013e318281b60a
- Richter KS, Bugge KR, Bromer JG, Levy MJ. Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil Steril. 2007;87(1):53-59. Available from: https://doi.org/10.1016/j.fertnstert.2006.05.064
- Takasaki A, Tamura H, Miwa I, Taketani T, Shimamura K, Sugino N. Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil Steril. 2010;93(6):1851-1858. Available from: https://doi.org/10.1016/j.fertnstert.2008.12.062
- Ng EH, Yeung WS, Ho PC. Endometrial and subendometrial vascularity are significantly lower in patients with endometrial volume 2.5 ml or less. Reprod Biomed Online. 2009;18:262–268. Available from: https://doi.org/10.1016/s1472-6483(10)60264-7
- Malhotra N, Malhotra J, Malhotra N, Rao JP, Mishra N. Endometrial receptivity and scoring for prediction of implantation and newer markers. Donald Sch J Ultrasound Obstet Gynecol. 2010;4(4):439–446. Available from: https://www.dsjuog.com/doi/pdf/10.5005/jp-journals-10009-1164
- Bassil S, Magritte JP, Roth J, Nisolle M, Donnez J, Gordts S. Uterine vascularity during stimulation and its correlation with implantation in in vitro fertilization. Hum Reprod. 1995;10:1497–1501. Available from: https://doi.org/10.1093/humrep/10.6.1497
- Hsieh YY, Chang FC, Tsai HD. Doppler evaluation of the uterine and spiral arteries from different sampling sites and phases of the menstrual cycle during the controlled ovarian hyperstimulation. Ultrasound Obstet Gynecol. pp. 192–196. Available from: https://doi.org/10.1046/j.1469-0705.2000.00196.x
- Jarvela IY, Sladkevicius P, Kelly S, Ojha K, Campbell S, Nargund G. Evaluation of endometrial receptivity during in vitro fertilization using three-dimensional power Doppler ultrasound. Ultrasound Obstet Gynecol. 2005;26:765–769. Available from: https://doi.org/10.1002/uog.2628
- Ng EH, Chan CC, Tang OS, Yeung WS, Ho PC. The role of endometrial and subendometrial blood flows measured by three-dimensional power Doppler ultrasound in the prediction of pregnancy during IVF treatment. Human Reprod. 2006;21:164–170. Available from: https://doi.org/10.1093/humrep/dei277
- Engmann L, Sladkevicius P, Agrawal R, Bekir J, Campbell S, Tan SL. The pattern of changes in ovarian stromal and uterine artery blood flow velocities during IVF treatment and its relationship with outcome of the cycle. Ultrasound Obstet Gynecol. 1999;13:26–33. Available from: https://doi.org/10.1046/j.1469-0705.1999.13010026.x
- Dechaud H, Bessueille E, Bousquet PJ, Reyftmann L, Hamamah S, Hedon B. Optimal timing of ultrasonographic and Doppler evaluation of uterine receptivity to implantation. Reprod Biomed Online. 2008;16:368–375. Available from: https://doi.org/10.1016/s1472-6483(10)60598-6
- Lesny P, Killick SR, Tetlow RL, Manton DJ, Robinson J, Maguiness SD. Ultrasound evaluation of the uterine zonal anatomy during in vitro fertilization and embryo transfer. Hum Reprod. 1999;14:1593–1598. Available from: https://doi.org/10.1093/humrep/14.6.1593
- Dickey RP, Olar TT, Curole DN, Taylor SN, Rye PH. Endometrial pattern and thickness associated with pregnancy outcome after assisted reproduction technologies. Hum Reprod. 1992;7(3):418-421. Available from: https://doi.org/10.1093/oxfordjournals.humrep.a137661
- Smith SK. Angiogenesis and implantation. Hum Reprod. 2000;15 Suppl 6:59-66.
- Gargett CE, Rogers PA. Human endometrial angiogenesis. Reproduction. 2001;121(2):181-186. Available from: https://doi.org/10.1530/rep.0.1210181
- Shimizu T, Jiang JY, Sasada H, Sato E. Changes of messenger RNA expression of angiogenic factors and related receptors during follicular development in gilts. Biol Reprod. 2002;67(6):1846-1852. Available from: https://doi.org/10.1095/biolreprod.102.006734
- Sugino N, Kashida S, Karube-Harada A, Takiguchi S, Kato H. Expression of vascular endothelial growth factor (VEGF) and its receptors in human endometrium throughout the menstrual cycle and in early pregnancy. Reproduction. 2002;123(3):379-387. Available from: https://doi.org/10.1530/rep.0.1230379
- Matsuoka-Sakata A, Tamura H, Asada H, Miwa I, Taketani T, Yamagata Y, et al. Changes in vascular leakage and expression of angiopoietins in the corpus luteum during pregnancy in rats. Reproduction. 2006;131(2):351-360. Available from: https://doi.org/10.1530/rep.1.00947
- Kashida S, Sugino N, Takiguchi S, Karube A, Takayama H, Yamagata Y, et al. Regulation and role of vascular endothelial growth factor in the corpus luteum during mid-pregnancy in rats. Biol Reprod. 2001;64(1):317-323. Available from: https://doi.org/10.1095/biolreprod64.1.317
- Sharkey AM, Day K, McPherson A, Malik S, Licence D, Smith SK, et al. Vascular endothelial growth factor expression in human endometrium is regulated by hypoxia. J Clin Endocrinol Metab. 2000;85(1):402-409. Available from: https://doi.org/10.1210/jcem.85.1.6229
- Goodman C, Jeyendran RS, Coulam CB. P53 tumor suppressor factor, plasminogen activator inhibitor, and vascular endothelial growth factor gene polymorphisms and recurrent implantation failure. Fertil Steril. 2009;92(2):494-498. Available from: https://doi.org/10.1016/j.fertnstert.2008.07.022
- Young SL. Oestrogen and progesterone action on endometrium: a translational approach to understanding endometrial receptivity. Reprod Biomed Online. 2013;27(5):497-505. Available from: https://doi.org/10.1016/j.rbmo.2013.06.010
- Garcia-Velasco JA, Acevedo B, Alvarez C, Alvarez M, Bellver J, Fontes J, et al. Strategies to manage refractory endometrium: state of the art in 201. Reprod Biomed Online. 2016;32(5):474-489. Available from: https://doi.org/10.1016/j.rbmo.2016.02.001
- Chen MJ, Yang JH, Peng FH, Chen SU, Ho HN, Yang YS. Extended estrogen administration for women with thin endometrium in frozen-thawed in vitro fertilization programs. J Assist Reprod Genet. 2006;23(7–8):337-342. Available from: https://doi.org/10.1007/s10815-006-9053-1
- Shen MS, Wang CW, Chen CH, Tzeng CR. New horizon on successful management for a woman with repeated implantation failure due to unresponsive thin endometrium: use of extended estrogen supplementation. J Obstet Gynaecol Res. 2013;39(5):1092-1094. Available from: https://doi.org/10.1111/j.1447-0756.2012.02070.x
- Demir B, Dilbaz S, Cinar O, Ozdegirmenci O, Dede S, Dundar B, et al. Estradiol supplementation in intracytoplasmic sperm injection cycles with thin endometrium. Gynecol Endocrinol. 2013;29(1):42-45. Available from: https://doi.org/10.3109/09513590.2012.705381
- Torres RF, Habana AE, Tansengco LG. The effect of estrogen supplementation on the endometrium and pregnancy rate among infertile women treated with clomifene citrate: a meta-analysis. Fertil Steril. 2005;84:S162-S163. Available from: http://dx.doi.org/10.1016/j.fertnstert.2005.07.399
- Cetinkaya K, Kadanali S. The effect of administering vaginal estrogen to clomiphene citrate stimulated cycles on endometrial thickness and pregnancy rates in unexplained infertility. J Turk Ger Gynecol Assoc. 2012;13(3):157-161. Available from: https://doi.org/10.5152/jtgga.2012.20
- Tourgeman DE, Slater CC, Stanczyk FZ, Paulson RJ. Endocrine and clinical effects of micronized estradiol administered vaginally or orally. Fertil Steril. 2001;75(1):200-202. Available from: https://doi.org/10.1016/s0015-0282(00)01640-x
- Weckstein LN, Jacobson A, Galen D, Hampton K, Hammel J. Low-dose aspirin for oocyte donation recipients with a thin endometrium: prospective, randomized study. Fertil Steril. 1997;68(5):927-930. Available from: https://doi.org/10.1016/s0015-0282(97)00330-0
- Wada I, Hsu CC, Williams G, Macnamee MC, Brinsden PR. The benefits of low-dose aspirin therapy in women with impaired uterine perfusion during assisted conception. Hum Reprod. 1994;9:1954–1957. Available from: https://doi.org/10.1093/oxfordjournals.humrep.a138366
- Kuo HC, Hsu CC, Wang ST, Huang KE. Aspirin improves uterine blood flow in the peri-implantation period. J Formos Med Assoc. 1997;96(4):253-257. Available from: https://pubmed.ncbi.nlm.nih.gov/9136511/
- Hurst BS, Bhojwani JT, Marshburn PB, Papadakis MA, Loeb TA, Matthews ML. Low-dose aspirin does not improve ovarian stimulation, endometrial response, or pregnancy rates for in vitro fertilization. J Exp Clin Assist Reprod. 2005;2:8. Available from: https://doi.org/10.1186/1743-1050-2-8
- Urman B, Mercan R, Alatas C, Balaban B, Isiklar A, Nuhoglu A. Low-dose aspirin does not increase implantation rates in patients undergoing intracytoplasmic sperm injection: a prospective randomized study. J Assist Reprod Genet. 2000;17(10):586-590. Available from: https://doi.org/10.1023/a:1026491426423
- Sher G, Fisch JD. Effect of vaginal sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil Steril. 2002;78(5):1073-1076. Available from: https://doi.org/10.1016/s0015-0282(02)03375-7
- Sher G, Fisch JD. Vaginal sildenafil (Viagra): a preliminary report of a novel method to improve uterine artery blood flow and endometrial development in patients undergoing IVF. Hum Reprod. 2000;15(4):806-809. Available from: https://doi.org/10.1093/humrep/15.4.806
- Di X, Gennings C, Bear HD, Graham LJ, Sheth CM, White KL Jr, et al. Influence of the phosphodiesterase-5 inhibitor, sildenafil, on sensitivity to chemotherapy in breast tumor cells. Breast Cancer Res Treat. 2010;124(2):349-360. Available from: https://doi.org/10.1007/s10549-010-0765-7
- Fetih AN, Habib DM, Abdelaal II, Hussein M, Fetih GN, Othman ER. Adding sildenafil vaginal gel to clomiphene citrate in infertile women with prior clomiphene citrate failure due to thin endometrium: a prospective self-controlled clinical trial. Facts Views Vis Obgyn. 2017;9(1):21-27. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC5506766/
- Zinger M, Liu JH, Thomas MA. Successful use of vaginal sildenafil citrate in two infertility patients with Asherman's syndrome. J Womens Health (Larchmt). 2006;15(4):442-444. Available from: https://doi.org/10.1089/jwh.2006.15.442
- Dehghani Firouzabadi R, Davar R, Hojjat F, Mahdavi M. Effect of sildenafil citrate on endometrial preparation and outcome of frozen-thawed embryo transfer cycles: a randomized clinical trial. Iran J Reprod Med. 2013;11(2):151-158. Available from: https://pubmed.ncbi.nlm.nih.gov/24639741/
- Pyriochou A, Zhou Z, Koika V, Petrou C, Cordopatis P, Sessa WC, et al. The phosphodiesterase 5 inhibitor sildenafil stimulates angiogenesis through a protein kinase G/MAPK pathway. J Cell Physiol. 2007 Apr;211(1):197-204. Available from: https://doi.org/10.1002/jcp.20929
- Eftekhar M, Rahmani E, Pourmasumi S. Evaluation of clinical factors influencing pregnancy rate in frozen embryo transfer. Iran J Reprod Med. 2014;12(7):513-518. Available from: https://pubmed.ncbi.nlm.nih.gov/25114675/
- LedeeBataille N, Olivennes F, Lefaix JL, Chaouat G, Frydman R, Delanian S. Combined treatment by pentoxifylline and tocopherol for recipient women with a thin endometrium enrolled in an oocyte donation programme. Hum Reprod. 2002;17(5):1249-53. Available from: https://doi.org/10.1093/humrep/17.5.1249
- Acharya S, Yasmin E, Balen AH. The use of a combination of pentoxifylline and tocopherol in women with a thin endometrium undergoing assisted conception therapies–a report of 20 cases. Hum Fertil (Camb). 2009;12(4):198-203. Available from: https://doi.org/10.3109/14647270903377178
- Cicek N, Eryilmaz OG, Sarikaya E, Gulerman C, Genc Y. Vitamin E effect on controlled ovarian stimulation of unexplained infertile women. J Assist Reprod Genet. 2012;29(4):325-328. Available from: https://doi.org/10.1007/s10815-012-9714-1
- Chiao TB, Lee AJ. Role of pentoxifylline and vitamin E in attenuation of radiation-induced fibrosis. Ann Pharmacother. 2005;39(3):516-522. Available from: https://doi.org/10.1345/aph.1e186
- Zackrisson U, Brannstrom M, Granberg S, Janson PO, Collins WP, Bourne TH. Acute effects of a transdermal nitric oxide donor on perifollicular and intrauterine blood flow. Ultrasound Obstet Gynecol. 1998;12(1):50-55. Available from: https://doi.org/10.1046/j.1469-0705.1998.12010050.x
- Ohl J, Lefebvre Maunoury C, Wittemer C, Nisand G, Laurent MC, Hoffmann P. Nitric oxide donors for patients undergoing IVF. A prospective, double-blind, randomized, placebo-controlled trial. Hum Reprod. 2002;17(10):2615-2620. Available from: https://doi.org/10.1093/humrep/17.10.2615
- Reynolds K, Khoury J, Sosnowski J, Thie J, Hofmann G. Comparison of the effect of tamoxifen on endometrial thickness in women with thin endometrium (<7 mm) undergoing ovulation induction with clomiphene citrate. Fertil Steril. 2010;93(6):2091-2093. Available from: https://doi.org/10.1016/j.fertnstert.2009.08.038
- Ke H, Jiang J, Xia M, Tang R, Qin Y, Chen ZJ. The effect of tamoxifen on thin endometrium in patients undergoing frozen-thawed embryo transfer. Reprod Sci. 2018 Jun;25(6):861-866. Available from: https://doi.org/10.1177/1933719117698580
- Licht P, Fluhr H, Neuwinger J, Wallwiener D, Wildt L. Is human chorionic gonadotropin directly involved in the regulation of human implantation? Mol Cell Endocrinol. 2007;269(1–2):85-92. Available from: https://doi.org/10.1016/j.mce.2006.09.016
- Eftekhar M, Rahmani E, Eftekhar T. Effect of adding human chorionic gonadotropin to the endometrial preparation protocol in frozen embryo transfer cycles. Int J Fertil Steril. 2012;6(3):175-176. Available from: https://pubmed.ncbi.nlm.nih.gov/24520435/
- Papanikolaou EG, Kyrou D, Zervakakou G, Paggou E, Humaidan P. Follicular HCG endometrium priming for IVF patients experiencing resisting thin endometrium. A proof of concept study. J Assist Reprod Genet. 2013;30(10):1341-1345. Available from: https://doi.org/10.1007/s10815-013-0076-0
- Davar R, Miraj S, Mojtahedi M. Farid. Effect of adding human chorionic gonadotropin to frozen thawed embryo transfer cycles with history of thin endometrium. Int J Reprod Biomed (Yazd). 2016;14(1):53-56. Available from: https://pubmed.ncbi.nlm.nih.gov/27141549/
- Wang CW, Horng SG, Chen CK, Wang HS, Huang HY, Lee CL, et al. Ovulation induction with tamoxifen and alternate-day gonadotrophin in patients with thin endometrium. Reprod Biomed Online. 2008;17(1):20-26. Available from: https://doi.org/10.1016/s1472-6483(10)60288-x
- Eftekhar M, Hosseinisadat R, Baradaran R, Naghshineh E. Effect of granulocyte colony stimulating factor (G-CSF) on IVF outcomes in infertile women: an RCT. Int J Reprod Biomed (Yazd). 2016;14(5):341-346. Available from: https://pubmed.ncbi.nlm.nih.gov/27326420/
- Tehraninejad E, Davari Tanha F, Asadi E, Kamali K, Aziminikoo E, Rezayof E. G-CSF Intrauterine for thin endometrium, and pregnancy outcome. J Family Reprod Health. 2015;9(3):107-112. Available from: https://pubmed.ncbi.nlm.nih.gov/26622308/
- Zenclussen AC, Hammerling GJ. Cellular regulation of the uterine microenvironment that enables embryo implantation. Front Immunol. 2015;6:321. Available from: https://doi.org/10.3389/fimmu.2015.00321
- Wurfel W, Santjohanser C, Hirv K, Buhl M, Meri O, Laubert I, et al. High pregnancy rates with administration of granulocyte colony-stimulating factor in ART-patients with repetitive implantation failure and lacking killer-cell immunglobulin-like receptors. Hum Reprod. 2010;25(8):2151-2152 author reply 2152. Available from: https://doi.org/10.1093/humrep/deq106
- Gleicher N, Vidali A, Barad DH. Successful treatment of unresponsive thin endometrium. Fertil Steril. 2011;95(6):2123.e13-7. Available from: https://doi.org/10.1016/j.fertnstert.2011.01.143
- Barad DH, Yu Y, Kushnir VA, Shohat-Tal A, Lazzaroni E, Lee HJ, et al. A randomized clinical trial of endometrial perfusion with granulocyte colony-stimulating factor in in vitro fertilization cycles: impact on endometrial thickness and clinical pregnancy rates. Fertil Steril. 2014;101(3):710-5. Available from: https://doi.org/10.1016/j.fertnstert.2013.12.016
- Eftekhar M, Sayadi M, Arabjahvani F. Transvaginal perfusion of G-CSF for infertile women with thin endometrium in frozen ET program: a non-randomized clinical trial. Iran J Reprod Med. 2014;12(10):661-6. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC4248151/
- Li Y, Pan P, Chen X, Li L, Li Y, Yang D. Granulocyte colony-stimulating factor administration for infertile women with thin endometrium in frozen embryo transfer program. Reprod Sci. 2014;21(3):381-5. Available from: https://doi.org/10.1177/1933719113497286
- Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco I, Correa do Amaral RJ, Granjeiro JM, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4(3):67. Available from: https://doi.org/10.1186/scrt218
- Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, et al. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8(1):1286-90. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC4358582/
- Fall M, Baranowski AP, Elneil S, Engeler D, Hughes J, Messelink EJ, et al. EAU guidelines on chronic pelvic pain. Eur Urol. 2010;57(1):35-48. Available from: https://doi.org/10.1016/j.eururo.2009.08.020
- Bodombossou-Djobo MM, Zheng C, Chen S, Yang D. Neuromuscular electrical stimulation and biofeedback therapy may improve endometrial growth for patients with thin endometrium during frozen-thawed embryo transfer: a preliminary report. Reprod Biol Endocrinol. 2011;9:122. Available from: https://doi.org/10.1186/1477-7827-9-122
- Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist Reprod. 2017;21(1):54-6. Available from: https://doi.org/10.5935/1518-0557.20170013
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13(1):87-101. Available from: https://doi.org/10.1093/humupd/dml045
- Cervello I, Mas A, Gil-Sanchis C, Peris L, Faus A, Saunders PT, et al. Reconstruction of endometrium from human endometrial side population cell lines. PLoS One. 2011;6(6):E21221. Available from: https://doi.org/10.1371/journal.pone.0021221
- Zhao J, Zhang Q, Wang Y, Li Y. Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reprod Sci. 2015;22(2):181-8. Available from: https://doi.org/10.1177/1933719114537715
- Jing Z, Qiong Z, Yonggang W, Yanping L. Rat bone marrow mesenchymal stem cells improve regeneration of thin endometrium in rat. Fertil Steril. 2014;101(2):587-94. Available from: https://doi.org/10.1016/j.fertnstert.2013.10.053
- Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81-5. Available from: https://doi.org/10.1001/jama.292.1.81
- Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082-6. Available from: https://doi.org/10.1634/stemcells.2006-0828
- Ikoma T, Kyo S, Maida Y, Ozaki S, Takakura M, Nakao S, et al. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol. 2009;201(6):608.e1-8. Available from: https://doi.org/10.1016/j.ajog.2009.07.026
- Morelli SS, Rameshwar P, Goldsmith LT. Experimental evidence for bone marrow as a source of nonhematopoietic endometrial stromal and epithelial compartment cells in a murine model. Biol Reprod. 2013;89(1):7. Available from: https://doi.org/10.1095/biolreprod.113.107987
- Hunter RK 2nd, Nevitt CD, Gaskins JT, Keller BB, Bohler HC Jr, LeBlanc AJ. Adipose-derived stromal vascular fraction cell effects on a rodent model of thin endometrium. PLoS One. 2015;10(12):e0144823. Available from: https://doi.org/10.1371/journal.pone.0144823
- Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman's syndrome. PLoS One. 2014;9(5):e96662. Available from: https://doi.org/10.1371/journal.pone.0096662
- Kilic S, Yuksel B, Pinarli F, Albayrak A, Boztok B, Delibasi T. Effect of stem cell application on Asherman syndrome, an experimental rat model. J Assist Reprod Genet. 2014;31(8):975-82. Available from: https://doi.org/10.1007/s10815-014-0268-2
- Gil-Sanchis C, Cervello I, Khurana S, Faus A, Verfaillie C, Simon C. Contribution of different bone marrow-derived cell types in endometrial regeneration using an irradiated murine model. Fertil Steril. 2015;103(6):1596-605.e1. Available from: https://doi.org/10.1016/j.fertnstert.2015.02.030
- Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman's syndrome. J Hum Reprod Sci. 2011;4(1):43-8. Available from: https://doi.org/10.4103/0974-1208.82360
- Singh N, Mohanty S, Seth T, Shankar M, Bhaskaran S, Dharmendra S. Autologous stem cell transplantation in refractory Asherman's syndrome: a novel cell based therapy. J Hum Reprod Sci. 2014;7(2):93-8. Available from: https://doi.org/10.4103/0974-1208.138864
- Santamaria X, Cabanillas S, Cervello I, Arbona C, Raga F, Ferro J, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087-96. Available from: https://doi.org/10.1093/humrep/dew042
- Schlaff WD, Hurst BS. Preoperative sonographic measurement of endometrial pattern predicts outcome of surgical repair in patients with severe Asherman's syndrome. Fertil Steril. 1995;63(2):410-3. Available from: https://doi.org/10.1016/s0015-0282(16)57379-8
- Jimenez PT, Schon SB, Odem RR, Ratts VS, Jungheim ES. A retrospective cross-sectional study: fresh cycle endometrial thickness is a sensitive predictor of inadequate endometrial thickness in frozen embryo transfer cycles. Reprod Biol Endocrinol. 2013;11:35. Available from: https://doi.org/10.1186/1477-7827-11-35
- Qublan H, Amarin Z, Al-Qudah M, Diab F, Nawasreh M, Malkawi S, et al. Luteal phase support with GnRH-a improves implantation and pregnancy rates in IVF cycles with endometrium of ≤7 mm on day of egg retrieval. Hum Fertil (Camb). 2008;11(1):43-7. Available from: https://doi.org/10.1080/14647270701704768
- Garrido-Gomez T, Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Vilella F, Simon C. Profiling the gene signature of endometrial receptivity: clinical results. Fertil Steril. 2013;99(4):1078-85. Available from: https://doi.org/10.1016/j.fertnstert.2012.12.005
- Diaz-Gimeno P, Horcajadas JA, Martinez-Conejero JA, Esteban FJ, Alama P, Pellicer A, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95(1):50-60.e1-15. Available from: https://doi.org/10.1016/j.fertnstert.2010.04.063
- Cruz F, Bellver J. Live birth after embryo transfer in an unresponsive thin endometrium. Gynecol Endocrinol. 2014;30(7):481-4. Available from: https://doi.org/10.3109/09513590.2014.900747
- Gargett CE, Nguyen HP, Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13(4):235-51. Available from: https://doi.org/10.1007/s11154-012-9221-9
- Du H, Naqvi H, Taylor HS. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev. 2012;21(18):3324-31. Available from: https://doi.org/10.1089/scd.2011.0193
- Cervello I, Gil-Sanchis C, Mas A, Faus A, Sanz J, Moscardo F, et al. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS One. 2012;7(1):e30260. Available from: https://doi.org/10.1371/journal.pone.0030260
- Issacs JD, Wells CS, William DB, Odem RR, Gast MJ, Strickler RC. Endometrial thickness is a valid monitoring parameter in cycles of ovulation induction with menotropins alone. Fertil Steril. 1996;65:262-6. Available from: https://doi.org/10.1016/s0015-0282(16)58082-0
- Check JH, Dietterich C, Lurie D, Nazari A, Chuong J. A matched study to determine whether low-dose aspirin without heparin improves pregnancy rates following frozen embryo transfer and/or affects endometrial sonographic parameters. J Assist Reprod Genet. 1998;15(10):579-82. Available from: https://doi.org/10.1023/a:1020373009043
- Check JH, Nowroozi K, Choe J, Dietterich C. Influence of endometrial thickness and echo patterns on pregnancy rates during in vitro fertilization. Fertil Steril. 1991;56(6):1173-5. Available from: https://doi.org/10.1016/s0015-0282(16)54736-0
- Bedaiwy MA, Abdelaleem MA, Hussein M, Mousa N, Brunengraber LN, Casper RF. Hormonal, follicular and endometrial dynamics in letrozole-treated versus natural cycles in patients undergoing controlled ovarian stimulation. Reprod Biol Endocrinol. 2011;9:83. Available from: https://doi.org/10.1186/1477-7827-9-83
- Gleicher N, Kim A, Michaeli T, Lee HJ, Shohat-Tal A, Lazzaroni E, et al. A pilot cohort study of granulocyte colony-stimulating factor in the treatment of unresponsive thin endometrium resistant to standard therapies. Hum Reprod. 2013;28(1):172-7. Available from: https://doi.org/10.1093/humrep/des370
- Lebovitz O, Orvieto R. Treating patients with “thin” endometrium - an ongoing challenge. Gynecol Endocrinol. 2014;30(6):409-14. Available from: https://doi.org/10.3109/09513590.2014.906571
- Kovacs P, Matyas S, Boda K, Kaali SG. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod. 2003;18(11):2337-41. Available from: https://doi.org/10.1093/humrep/deg461
- Gelbaya TA, Kyrgiou M, Li TC, Stern C, Nardo LG. Low-dose aspirin for in vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update. 2007;13(4):357-64. Available from: https://doi.org/10.1093/humupd/dmm005