More Information
Submitted: April 10, 2025 | Approved: April 18, 2025 | Published: April 21, 2025
How to cite this article: Elsammani M, Elhawari S, Murad G, Nasr K, Abdelgader A, Suliman H, et al. Types and Outcomes of Diagnostic Measures provided for women Presented with Postmenopausal Bleeding. Clin J Obstet Gynecol. 2025; 8(2): 030-036. Available from:
https://dx.doi.org/10.29328/journal.cjog.1001186
DOI: 10.29328/journal.cjog.1001186
Copyright license: © 2025 Elsammani M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Postmenopausal; Bleeding; Hysteroscopy; Endometrial hyperplasia; Dilation curettage; Endometrial cancer
Abbreviations: D&C: Dilatation and Curettage; DM: Diabetes Mellitus; EC: Endometrial Cancer; EH: Endometrial Hyperplasia; ET: Endometrial Thickness; EV: Endometrial Volume; HRT: Hormonal Replacement Therapy; IVF: In Vitro Fertilization; HTN: Hypertension; PCOS: Polycystic Ovarian Syndrome; PMB: Postmenopausal Bleeding; UK: United Kingdom; USS: Ultrasound Scan; TVS: Transvaginal Scan
Types and Outcomes of Diagnostic Measures provided for women Presented with Postmenopausal Bleeding
Malaz Elsammani1, Sahar Elhawari2, Gawahir Murad1, Khairi Nasr2, Alla Abdelgader1, Hajar Suliman3, Baharelden Abuobida1, Bashir Abdeen1 and Awadalla Abdelwahid3*
1Specialist of Obstetrics and Gynecology, MD, Sudan Medical Specialization Board (SMSB), Sudan
2Consultant of Obstetrics and Gynecology, Assistant Professor, Faculty of Medicine, Khartoum University, Sudan
3Consultant of Obstetrics and Gynecology, Assistant Professor, Faculty of Medicine, Al Neelain University, Sudan
*Address for Correspondence: Awadalla Abdelwahid, Assistant Professor of Obstetrics and Gynecology, Faculty of Medicine, Al Neelain University, Khartoum, Sudan, Email: [email protected]
Background: Postmenopausal bleeding (PMB) is bleeding from the genital tract after 12 months of amenorrhea in a woman over the age of 50, or 24 months if below the age of 50 years, in 10% of women presented with PMB, the cause is endometrial cancer.
Purpose: To assess the clinical presentation types and outcomes of diagnostic measures provided for women presenting with PMB at Saad Abu-Alela Hospital, Khartoum, Sudan.
Methodology: It was a descriptive, cross-sectional study conducted at Saad Abu-Alela Teaching Hospital in the period from January to December 2022.
An interview questionnaire was used for data collection. Fifty-nine (59) postmenopausal women were included in this study, age, parity, risk factors, duration of bleeding, duration of menopause, ultrasound findings, and hysteroscopy findings were recorded.
Results: The majority of study participants were aged between 50-54 years, menopause duration was most 1-4 years, most of the participants were educated and medically free, DM and HTN, and most of the participants were multiparous. Duration of PMB ranged between weeks in a third of cases and up to more than a year in some cases, amount of bleeding was mild in more than half. Ultrasound is used to assess the endometrial thickness and other findings, also hysteroscopy and biopsy or Dilation and curettage and hysterectomy.
Conclusion: The ultrasound and endometrial biopsy via inpatient hysteroscopy and dilatation and curettage were the best tools for evaluation of (PMB), benign conditions were the most frequent outcome and endometrial cancer.
Postmenopausal Bleeding (PMB), defined as uterine bleeding occurring at least a year following menstrual cessation, is a prevalent clinical condition with an incidence of 10% soon after menopause [1]. Patients with PMB have a 10% - 15% likelihood of developing endometrial carcinoma [2-6]. As a result, the clinical management of PMB necessitates prompt and efficient evaluation to rule out cancer in the genital tract or precancerous lesions of the endometrium. Despite this, vaginal atrophy and benign focal lesions, such as endometrial polyps and fibroids, are common. The incidence of endometrial polyps and hyperplasia is estimated to be 40% [2,3]. PMB is generally attributed to an intrauterine source, but it may originate from the vulva, vagina, cervix, and fallopian tubes, or may be connected to ovarian pathology [7]. Ninety % of women with endometrial carcinoma present with vaginal bleeding [7]. Endometrial cancer is the most frequent gynecological malignancy [4]. Factors increasing the risk include obesity, unopposed estrogens, polycystic ovary syndrome, and nulliparity. Patient-specific characteristics, including age, time elapsed since menopause, obesity, hypertension, and diabetes mellitus, are known risk factors for endometrial carcinoma. At present, policy is based not on these risk factors but on endometrial thickness [7].
A history and examination are the first steps in the diagnostic process for PMB. The purpose of the history is to determine the type of bleeding, risk factors, precipitating factors, cervical smear history, weight loss, changes in bowel habits, and medication history, including HRT and compliance. After that, a clinical examination is performed to rule out cervical polyps, cervicitis, vulval and vaginal tumors/ulcerations, genital tract atrophy, abdominopelvic mass, and cervical carcinoma. In addition to a general examination and a bimanual pelvic assessment to evaluate anesthesia and surgical suitability. The patient's condition and suitability for surgery and anesthesia are then evaluated through a general investigation. For women with PMB, transvaginal sonography (TVS) is currently the first line of investigation [8,9]. Because TVS is relatively non-invasive, it is more widely accepted. Compared to premenopausal women, postmenopausal women have significantly thinner mean endometrial thicknesses. As t increases, the probability of endometrial cancer is also increased.
Postmenopausal women have significantly thinner mean endometrial thicknesses than premenopausal women [10]. With increasing endometrial thickness, the chance of endometrial cancer developing rises. Patients should have more invasive testing done if their endometrial thickness is increased [11-13]. The following methods can be used for sampling: outpatient endometrial aspiration e. dilatation, curettage, hysteroscopy, and pipelle. Outpatient biopsy by endometrial aspiration; e. A. D and C and anesthesia-related complications are avoided with pipelle and vabra aspirators. Since hysteroscopy can identify focal lesions like polyps that blind sampling might overlook, it may be required in conjunction with additional endometrial assessment if intrauterine structural abnormalities like polyps are suspected on TVS or endometrial biopsy. With the use of tiny hysteroscopes, diagnostic hysteroscopy can be performed in an outpatient setting without the use of vaginal instruments or anesthesia [14-19].
This study employed a cross-sectional, descriptive design and was conducted at an institution between January and December 2022. The study was conducted at Saad Abu-Alela Hospital, a teaching hospital specializing in obstetrics and gynecology at the University of Khartoum. Maternal and child health care, obstetrics and gynecology ultrasound, natural childbirth, obstetrics and gynecology operations, fertility and In vitro fertilization (IVF) services, colposcopy and cervical cancer detection, laparoscopy and hysteroscopy, maternal intensive care, neonatal unit, scientific research, training of medical and higher nursing students, training of obstetrics and gynecology, anesthesiology, neonatology, emergency medicine, family medicine registrars, and house officers are among the services offered by the hospital.
Study population
This study included all patients who were seen in the gynecological casualty at Saad Abu-Alela Hospital and who met the following requirements: they had to have PMB, accept to participate in the study, be recruited from the study area within the allotted time frame, and not be critically ill women.
Convenience sampling was used, and we included all patients who were seen in the gynecological casualty with PMB who were available during the data collection period, and who consented to participate.
Methods
All postmenopausal women had their complete medical history, age, parity, length of menopause, bleeding duration, risks of endometrial cancer and hyperplasia, and uterus position, fixity, pelvic mass, fibroid, polyps, cervical mass, and Douglas pouch examined together with their general, abdominal, and pelvic examinations. For endometrial thickness, fibroids, polyps, cancer, and ovarian masses, women were offered routine tests and ultrasound scans.
The endometrial thickness cutoff for postmenopausal women was set at > 5 mm. A biopsy from the endocervical canal is performed for D&C women, followed by cervical dilatation up to 7-8 Hegar. The women were advised and gave their consent for a hysterectomy, a hysteroscopy, a biopsy, and dilatation and curettage.
To remove endometrial polyps, ring forceps were employed. Using a sharp curette, the fundus, posterior wall, anterior wall, and final right and left lateral walls were curettage. The sample was put in 10% formalin by the pathologist for histopathological examination.
Hysteroscopic examination
A vaginal USG was conducted to assess the uterus's size, consistency, mobility, and bilateral adnexa while the patient was under general anesthesia and in the dorsal lithotomy position. The hysteroscopy was performed by an operator who was blind to the ultrasonography results. The procedure of hysteroscopy was conducted in an appropriately sized and fully equipped treatment room dedicated to hysteroscopy, private and patient-friendly, to alleviate anxiety with a separate, and ideally adjoining, changing area with a toilet. Adequate resuscitation facilities were available, along with a comfortable recovery area.
Access to on-site decontamination facilities of an appropriate standard was ensured.
Appropriate staffing levels were available to advocate for the women during the procedure, providing reassurance, explanation, and support. Oral pain relief was provided one hour before their scheduled appointment. Besides explaining details about what the procedure entails, benefits and risks (including pain), and alternative options.
Verbal and written informed consent were given to the woman during their appointment before the hysteroscopy was performed.
Cervical preparation with prostaglandins was not done. Hysteroscopes of 3.5 mm in outer diameter were used for diagnostic procedures by a trained clinician using warm saline infusion at the lowest possible pressure as distending media. The cervix was not routinely dilated before the procedure nor vaginum speculum used to minimize the pain. Documentation after the procedure in standardized proforma was ensured. A panoramic hysteroscopy with a rigid continuous flow was used.
With a 12° fiberoptic lens, a 7 mm outer sheath diameter, and a 25 cm length, it was a rigid continuous flow panoramic hysteroscopy. Continuous uterine distention was achieved by attaching plastic bags of uroflow or sorbitol mannitol solution. Through the use of a monometrically controlled pneumatic cuff, the infusion pressure was increased to between 100- and 130-mm HG dot. The process was captured on a single-chip video, which the operator could view on a monitor. Following the telescope's insertion into the cavity, the fundus, anterior, posterior, and lateral walls of the uterus were all inspected to visualize the uterotubal junctions. They get thicker.
Hysteroscopic evaluation
While each patient was under general anesthesia and blind to the ultrasound results, a single, trained operator conducted a diagnostic hysteroscopy on them. We employed a 25 cm long, inflexible, constant-flow panoramic hysteroscopy with a 6 mm outer sheath. Attaching plastic bags containing a 1% - 5% saline or glycine solution to dual infusion tubing allowed for continuous uterine distention. The cavity was examined for polyploidy endometrium, myomas, masses, or polyps.
Data collection
A thorough, structured, closed-ended questionnaire was employed as one of the data collection tools. The researcher and registrars interviewed the women who gave their verbal and written consent to participate in the study in order to gather the data.
Data entry, analysis and presentation
SPSS 21 version was used to enter, clean, and analyze data using descriptive statistics in the form of graphs and frequency tables with %ages. For quantitative data, means and standard deviations are displayed with pertinent graphical representation. Variables under investigation include postmenopausal women's age, length of menopause, education, parity, length of PMB, endometrial thickness in USS, other USS findings, biopsy technique, and histopathology findings.
Ethical considerations
Saad Abu-Alela Hospital's administrative authority and the Sudan Medical Specialization Board approved the study on 10 April, 2023.NO: (10439) and data and information were used exclusively for research.
Privacy concerns were taken into account. Respondents were informed of the purpose of the study, and participation was entirely voluntary. Additionally, written consent was given to each of them. Every participant had the freedom to leave at any time.
The current study was conducted between January 2022 and December 2022 on 59 postmenopausal women who had postmenopausal bleeding and were recruited from Saad Abu-Alela Hospital. The demographic characteristics of postmenopausal women. Table 1 found the age distribution of the study participants, majority of them 27(46.7%) were between (50-54) years, followed by 12(20%) at (55-59) years, 8 (13.3%) at 65-69 years, 6(10%) at 60-64 years, 4(6.7%) at (70-74) years and 2(3.3%) were above 75 years.
| Table 1: Demographic Characteristics of Postmenopausal Women (N = 59). | |||
| Demographic characteristics | Frequency | Percent | p - value |
| Age 50-54 | 27 | 45.8% | |
| 55-59 | 12 | 20.3% | |
| 60-64 | 8 | 13.5% | |
| 65-69 | 6 | 10.2% | 0.01 |
| 70-74 | 4 | 6.8% | |
| 75-79 | 2 | 3.4% | |
| Education Illiterate | 11 | 18.6% | |
| Primary school | 15 | 25.5% | |
| Secondary school | 10 | 16.9% | 0.02 |
| University | 21 | 35.6% | |
| Postgraduates | 7 | 11.9% | |
| Parity Nulliparous | 27 | 45.7% | |
| Multiparous | 25 | 42.4% | 0.01 |
| Grand multiparous | 7 | 11.9% | |
| Total | 59 | 100% | |
Duration of participants' menopause, was 1 year in only 2(3.4%) of cases, majority were between (2-5) years in 29(49.2%), (5-9) years in10 (16.9%) of cases, (10-14) years in 7(11.9%) of cases and more than 15 years in 11(18.6) of cases.
The distribution of study participants according to their educational level, 2(3.4%) of them attained postgraduate education 21(35.6%) of them were university graduates, primary school 15(25.5%), secondary school 10(16.9%) and 11 (18.6.0%) did not go to schools.
In regard to the distribution of the study participants according to parity, 7(11.9%) were nulliparous, while 27(45.7%) were multiparous and 25(42.4%) were grand multiparous.
The clinical characteristics of postmenopausal women include risk factors, duration of menopause, and duration of bleeding (Table 2).
| Table 2: Clinical Characteristics of Postmenopausal Women (N = 59). | |||
| Clinical characteristics | Frequency | Percent | p - value |
| Menopause duration ≤ 1 year | 2 | 3.4% | |
| ≥ 1-4 year | 29 | 49.2% | |
| 5-9 year | 10 | 16.9% | |
| 10-14 year | 7 | 11.9% | 0.02 |
| ≥15 year | 11 | 18.6% | |
| Risk factors No risk | 3 | 5.1% | |
| Hypertension | 22 | 37.3% | |
| Diabetes | 17 | 28.8% | 0.01 |
| PCO | 1 | 1.7% | |
| Obesity | 16 | 27.1% | |
| Severity Spotting | 4 | 6.8% | |
| Mild | 31 | 52.6 | 0.01 |
| Moderate | 14 | 23.7 | |
| Severe | 10 | 16.9 | |
| Duration ≤ 1 month | 18 | 30.5% | |
| 1 - 3 months | 14 | 23.7 | |
| 4 - 6 months | 15 | 25.4% | 0.01 |
| 7 - 12 months | 3 | 5.1 | |
| > 12 month | 9 | 15.3 | |
| Total | 59 | 100% | |
The risk factors for PMB, 3(5.1%) were medically free, while 1(1.70%) were diagnosed as PCOS 22(37.3%) suffers hypertension, 17(28.8%) were diabetic and16 (27.1%) were obese.
The duration of postmenopausal bleeding ranged from less than one-month in18 (30.5%), (1-3) months in 14(23.7%), (4-6) months in 15(25.4%), 3(5.1%) (7-12) months and 9(15.3%) has bleeding that lasted more than one year.
All women were offered a pelvic ultrasound to measure the endometrial thickness by ultrasound, it was less than 3 mm in 3(5.1%), while it was more than 15 mm in 19(32.2%). The rest ranged between 3-4 mm in 16(27.1%), 5-9 mm in 10(16.9%) and 10-14 mm in 11(18.6%) (Table 3).
| Table 3: Ultrasound Endometrial Thickness of Postmenopausal Women (N = 59). | ||
| Endometrial thickness | Frequency | Percent |
| < 3 mm | 3 | 5.1% |
| 3 - 4 mm | 16 | 27.1% |
| 5 - 9 mm | 10 | 16.9 |
| 10 - 14 mm | 11 | 18.6 |
| 15 mm and more | 19 | 32.2 |
| Total | 59 | 100% |
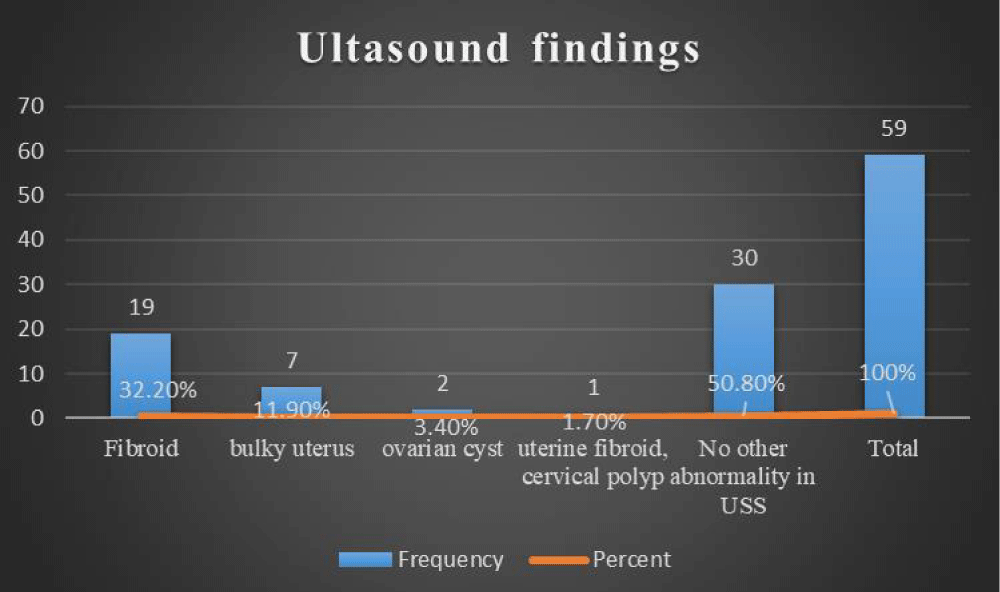
Also, ultrasound findings other than endometrial thickness in the study participants, fibroids were found in 19(32.2%), bulky uterus 7(11.9%), ovarian cyst in 2(3.4%), cervical polyp1(1.7%) while in 30(50.8%) no abnormality was reported by ultrasound (Figure 1).
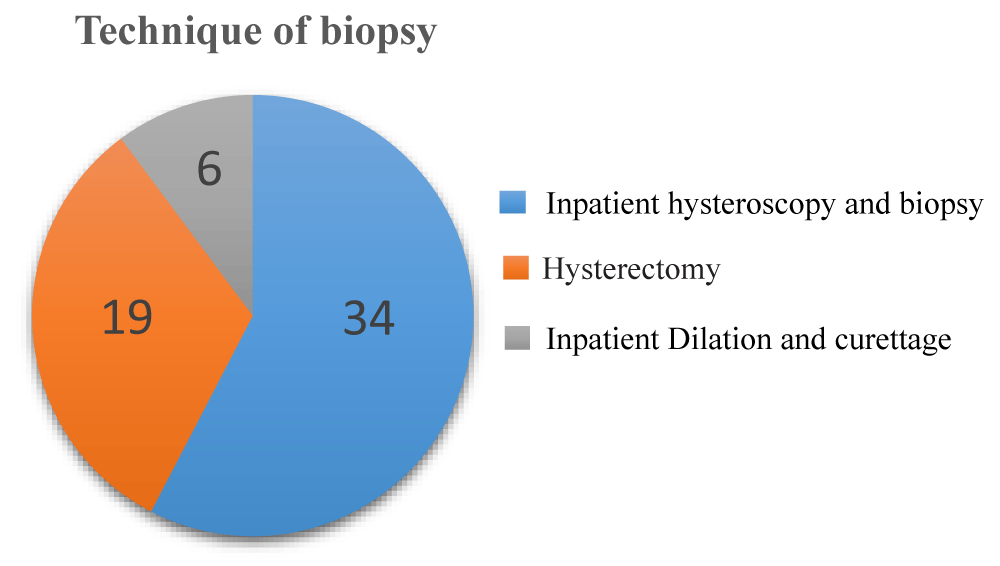
Figure 1: Technique of Biopsy of Postmenopausal Women (N = 59).
Diagnostic investigations procedure was undergone for the study population for biopsy and histology, the majority 34(57.6%) were inpatient hysteroscopy and biopsy, while hysterectomy in 19(32.2%) and inpatient dilatation and curettage in 6(10.2%) (Figure 2).
Figure 2: Ultrasound finding of Postmenopausal Women (N=59).
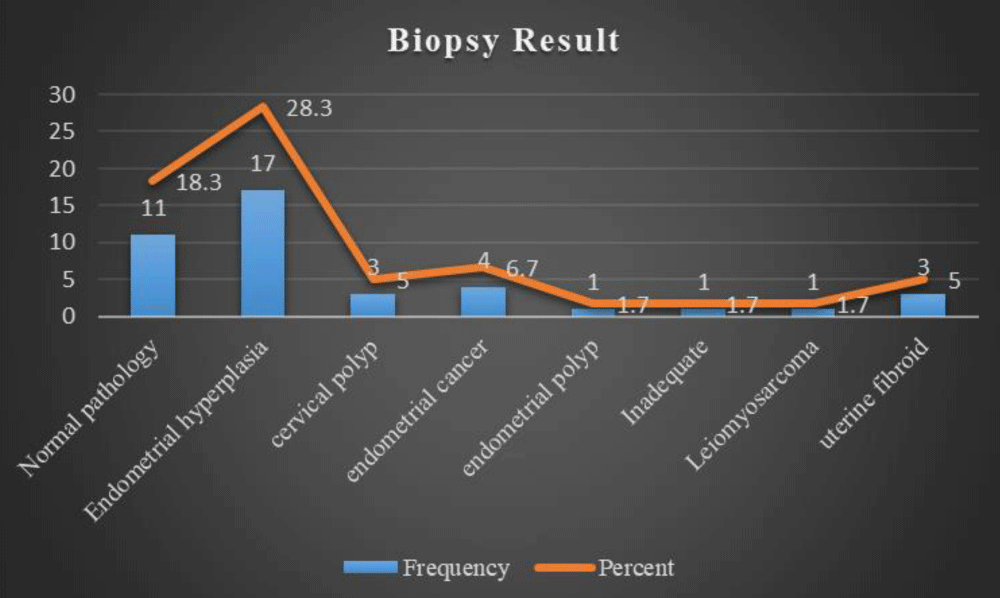
The study participants' results of the biopsy showed endometrial hyperplasia was found in 17(28.8%), while a normal pathology was found in 11(18.6%), cervical polyps in 3(5.1%), endometrial cancer in 4(6.8%) and uterine fibroid in 3(5.1%) (Figure 3).
Figure 3: Biopsy Result of Postmenopausal Women.
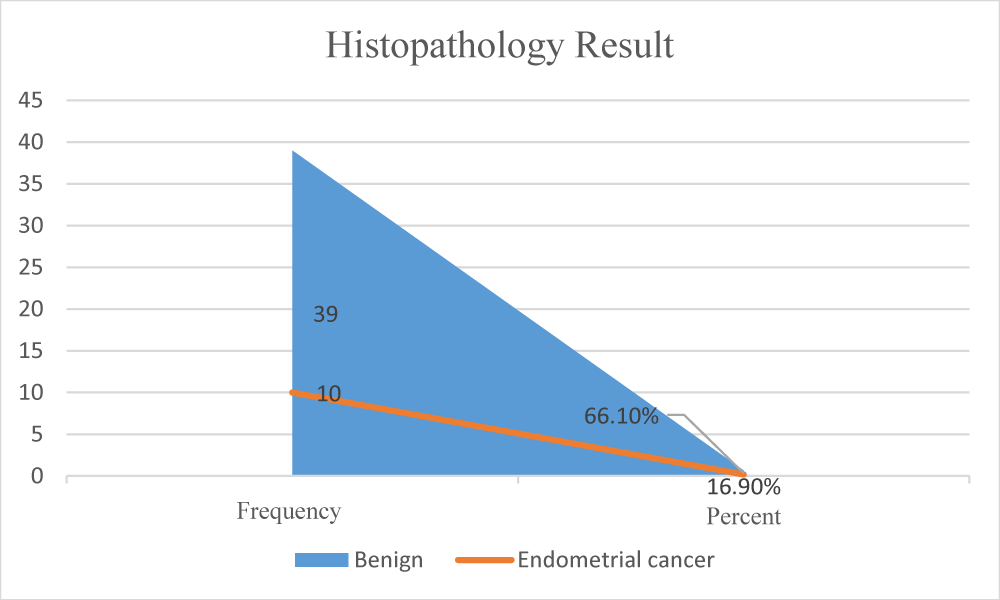
Post-menopausal women underwent hysterectomy their histopathology findings for hysterectomy samples, showed benign pathology in the majority 39(66.1%) and endometrial cancer was present in 10(16.9%) of the cases. (Figure 4).
Figure 4: Histopathology Result of Postmenopausal Women.
Postmenopausal bleeding is a serious common health problem worldwide, which should be addressed seriously because in 10% of women presented with PMB, the cause is endometrial cancer, so their access to diagnostics must be deemed urgent. This study was designed to identify the presentations and diagnoses of patients who presented with postmenopausal bleeding, as well as the diagnostic care they had received, which helps healthcare providers maintain and provide proper care and clinical practice to assist patients with postmenopausal bleeding. The total number of women presented with PMB and included in this study was 59, ages of women ranged between (50-80) years, majority of women aged 27(46.7%) were between (50-54) years which is similar to the study conducted in India on clinical and histopathological characteristics in women with postmenopausal bleeding [20,21] and less than a study in the Netherlands which found mean age (55-64) years [22].
The duration of menopause was the majority of cases, between 2-5 years (49.2%). This finding is in concordance with the results reported in a cross-sectional study in the UK [23] and another study on the endocrine identification of menopause in Sudan [24].
The common risk factors identified in this study for PMB were, polycystic ovary syndrome, diabetes, hypertension, and obesity which were found similar to [26], Another author also observed that an increase in BMI was associated with an increase in endometrial thickness [27]. A few other studies reported no association between hypertension and endometrial thickness [28,29], and also study by Syeda, et al. [30] found obesity is the most risk factor.
All women presented with postmenopausal bleeding in this study offered ultrasound scans which is a routine method of screening in patients with suspected intrauterine pathology [31] because it is low cost, none invasive reproducibility, and absence of complications, the thickness of more than 4mm used to cut off, the endometrial thickness of more than 5 was used as an indicator for endometrial pathology in another study [9], Turkey study uses the ratio of endometrial thickness to the of the uterine wall full thickness and the subcutaneous adipose tissue which it found significant in endometrial cancer and endometrial hyperplasia [32].
Pelvic ultrasound (3D) offers good diagnostic tools in a postmenopausal woman as it has a higher capacity to outline outer uterine walls and inner endometrial abnormalities, than (2D), especially uterine fibroids, cervical polyps, endometrial polyps, and ovarian cysts, our study finding were similar to Egypt study [33] which found ultrasound is sensitive in the detection of fibroid, polyps.
The uterus was previously only accessible by the blind dilation and curettage (D and C) procedure but can now be evaluated by clinicians thanks to the advent of intrauterine endoscopy. Despite numerous studies demonstrating that hysteroscopy is superior to D&C, its application is still not fully understood [34], this study included endometrial and endocervical biopsies inpatient hysteroscopy, and biopsy was undertaken in 34(57.6%) since hysteroscopy is a safe and extremely sensitive diagnostic technique, it is the best way to assess patients who are experiencing postmenopausal bleeding and accurate diagnosis is achieved when hysteroscopy and endometrial biopsy are used together [35], a woman not candidate for hysteroscopy or D&C offered hysterectomy and inpatient dilatation and curettage was undertaken in 10% of postmenopausal women.
Atrophic endometritis, endometrial hyperplasia, and endometrial cancer are the three primary causes of PMB [35]. Blind endometrial biopsies might miss endometrial polyps, which could lead to an underdiagnosis of the condition after menopause [36].
Endometrial hyperplasia was observed in (28.3%) of the biopsies, and this number was higher than that reported in the literature as the incidence of EH in the postmenopausal age group was about 13% - 14% [37]. Also, higher than Indian study of correlation risk factors of endometrial hyperplasia and cancer with a clinicopathological evaluation of postmenopausal bleeding which found 11.8% [38] in this study Carcinoma of endometrium was the second most common finding which was seen in (6.7%) of biopsies, cervical polyps in (5.1%) of cases, and uterine fibroid in (5.1%) of cases. Other, less common histopathological findings comprised (5%) of cases and included one case of endometrial polyp, one case of leiomyosarcoma, and one case of uterine fibroid. (18.3%) have normal endometrial histology and (31.7%) of study participants haven’t had biopsies taken which is comparable with contributing factors for postmenopausal bleeding study [39].
Histopathology findings for hysterectomy samples showed benign pathology in the majority (66.1%) of the cases and endometrial cancer was present in (16.9%) of the cases which is comparable with the Lahore study which found hyperplasia in (4.5%) of cases, atypical hyperplasia in (1.8%) of cases and endometrial carcinoma in (4.07%) of cases also there was no statistical difference was noted in different age groups [40].
Limitation of the study
It is significant to mention that one of the obstacles encountered in this study was the lack of a single operator responsible for the entire study population. Additionally, the research was carried out within a solitary hospital, thereby constraining the scope of its generalizability.
One of the strengths of this study it is the first study that highlights this health problem and the registrar, and specialist who participated in this study found an opportunity to have training in hysteroscopy.
Dilatation and curettage, endometrial biopsy via inpatient hysteroscopy, and ultrasound were among the diagnostic techniques used. In postmenopausal women with irregular uterine bleeding, endometrial cancer is also significant. The study concluded that transvaginal ultrasonography is a useful screening method for identifying endometrial abnormalities, although benign conditions were the most common outcome. Its high sensitivity improves its role in initial diagnostic evaluation by ensuring the early detection of cancerous cases, by hysteroscopy biopsy and D&C.
Recommendations
Training of health professionals in hysteroscopy will improve the patient's care and enable detection of precancerous lesions which reduce morbidity and mortality.
The introduction of outpatient endometrial sampling techniques and malleable 5 mm hysteroscopy will reduce patients' suffering, and hospital costs, and expedite the time taken to obtain the histology results.
We would like to thank Prof. Abedelbagi Elzien for his supervision, guidance, and help for this research.
Availability of data and materials
Data sets used and analyzed in the current study are available from the corresponding author on a reasonable request.
Declarations
Ethical approval and consent to participate: This study was conducted at Saad Abu-Alela Teaching Hospital and approved by the Ethics Committee of The Sudan Medical Specialization Board on 10 April, 2023.NO: (10439).
All participants signed an informed consent form for inclusion in the study.
- Astrup K, Olivarius NDF. Frequency of spontaneously occurring postmenopausal bleeding in the general population. Acta Obstet Gynecol Scand. 2004;83(2):203-207. Available from: https://doi.org/10.1111/j.0001-6349.2004.00400.x
- Timmermans A, Gerritse MB, Opmeer BC, Jansen FW, Mol BW, Veersema S. Diagnostic accuracy of endometrial thickness to exclude polyps in women with postmenopausal bleeding. J Clin Ultrasound. 2008;36(5):286-290. Available from: https://doi.org/10.1002/jcu.20415
- Buchanan C, Robinson M, Macdonald MC. Endometrial cancer rate in Hormone replacement therapy users with postmenopausal bleeding: Retrospective cohort study. Post Reprod Health. 2022;28(3):143–8. Available from: https://doi.org/10.1177/20533691221116171
- Xue H, Shen WJ, Zhang Y. The pathological pattern of endometrial abnormalities in postmenopausal women with bleeding or thickened endometrium. World J Clin Cases. 2022;10(7):2159–65. Available from: https://doi.org/10.12998/wjcc.v10.i7.2159
- Jones ER. Developing Tests for Endometrial Cancer Detection (DETECT) [dissertation]. Manchester (UK): The University of Manchester; 2023. Available from: https://research.manchester.ac.uk/en/studentTheses/developing-tests-for-endometrial-cancer-detection-detect
- Saccardi C, Spagnol G, Bonaldo G, Marchetti M, Tozzi R, Noventa M. New light on endometrial thickness as a risk factor of cancer: what do clinicians need to know? Cancer Manag Res. 2022;13:1331–40. Available from: https://doi.org/10.2147/cmar.s294074
- Teasdale A, Gale K, Holloway D. Menstrual and hormonal dysfunction. In: Nursing Management of Women’s Health: A Guide for Nurse Specialists and Practitioners. 2019;7-47.
- Swetha RN, Rao MP, Penumaka R. Evaluation of Endometrial Thickness by Transvaginal Sonography in Postmenopausal Women with Bleeding: A Cross-sectional Study. J Clin Diagn Res. 2024;18(1):QC12–7. Available from: https://doi.org/10.7860/JCDR/2024/67553.18975
- Amer ASS, Diab YMS, Fotouh AMMAE. Comparison Between Endometrial Thickness by TVS And Endometrial Histopathology in Postmenopausal Women. Al-Azhar Int Med J. 2024;5(3):48. Available from: https://aimj.researchcommons.org/cgi/viewcontent.cgi?article=2335&context=journal
- Stephenson RG, Cathcart DB. The Physical Therapist’s Guide to Women's Pelvic, Perinatal, and Reproductive Health. New York: Routledge; 2025. Available from: https://www.routledge.com/The-Physical-Therapists-Guide-to-Womens-Pelvic-Perinatal-and-Reproductive-Health/Stephenson-Cathcart/p/book/9781630917869?srsltid=AfmBOoq4rvJwau1sSYgwA4qQ-9pNQf4mWlLKGgeM4Iom3Kf1-YxPYcDc
- Baker-Rand H, Kitson SJ. Recent advances in endometrial cancer prevention, early diagnosis, and treatment. Cancers. 2024;16(5):1028. Available from: https://doi.org/10.3390/cancers16051028
- Doll KM, Pike M, Alson J, Williams P, Carey E, Stürmer T, et al. Endometrial thickness as diagnostic triage for endometrial cancer among Black individuals. JAMA Oncol. 2024;10(8):1068–76. Available from: https://doi.org/10.1001/jamaoncol.2024.1891
- Cabrera S, de la Calle I, Baulies S, Gil-Moreno A, Colas E. Screening strategies to improve early diagnosis of endometrial cancer. J Clin Med. 2024;13(18):5445. Available from: https://doi.org/10.3390/jcm13185445
- Vitale SG, Buzzaccarini G, Riemma G, Pacheco LA, Sardo ADS, Carugno J, et al. Endometrial biopsy: Indications, techniques, and recommendations. An evidence-based guideline for clinical practice. J Gynecol Obstet Hum Reprod. 2023;52(6):102588. Available from: https://doi.org/10.1016/j.jogoh.2023.102588
- De Silva PM. The diagnosis and treatment of endometrial pathology in the outpatient setting [dissertation]. Birmingham (UK): University of Birmingham; 2023.
- Sutedja MN, Cristobal RJGV, Ferraris MCCC, Alcaraz CJL, Gonzales-Acantilado GV. The Comparative Efficacy of Endometrial Biopsy using Pipelle and Diagnostic Dilatation & Curettage in Mariano Marcos Memorial Hospital and Medical Center. Bioscientia Med. 2023;7(10):3652–7. Available from: https://doi.org/10.37275/bsm.v7i10.871
- Demirkiran F, Yavuz E, Erenel H, Bese T, Arvas M, Sanioglu C. Which is the best technique for endometrial sampling? Aspiration (pipelle) versus dilatation and curettage (D&C). Arch Gynecol Obstet. 2012;286:1277–88. Available from: https://doi.org/10.1007/s00404-012-2438-8
- Abo El-fath A, Alnemr A, Eid E, Harb OA. Diagnostic Value of Pipelle, Curretage Biopsy and Hysteroscopic Biopsy in Cases of Postmenopausal Bleeding. Zagazig Univ Med J. 2024;30(1.7):4019–32. Available from: http://dx.doi.org/10.21608/zumj.2024.264675.3129
- Waris A, Bibi T, Ch S, Wajid A, Jaweria F, Munir MW. Comparison of diagnostic accuracy of endometrial sampling techniques; D&C and Pipelle sampling in patients of endometrial hyperplasia. Res Med Sci Rev. 2025;3(1):976–87. Available from: https://thermsr.com/index.php/Journal/article/view/472
- Gupta A. Clinical and histopathological characteristics in women with Postmenopausal bleeding. Age (years). 45(49):10.
- Purushotham MK. Clinicopathological Study of Postmenopausal Bleeding [Master's thesis]. Bangalore: Rajiv Gandhi University of Health Sciences (India); 2018.
- Jajou R, van Puijenbroek EP, Veldkamp R, Overbeek JA, van Hunsel FP, Kant AC. General practitioner consultation for postmenopausal bleeding after COVID‐19 vaccination—a self‐controlled cohort study. Br J Clin Pharmacol. 2025. Available from: https://doi.org/10.1002/bcp.70045
- Ewies AA, Musonda P. Managing postmenopausal bleeding revisited: what is the best first line investigation and who should be seen within 2 weeks? A cross-sectional study of 326 women. Eur J Obstet Gynecol Reprod Biol. 2010;153(1):67–71. Available from: https://doi.org/10.1016/j.ejogrb.2010.06.009
- Khalid MM. Endocrine identification of menopausal status of Sudanese women [Internet]. 2010 [cited 2025 Apr 18]. Available from: https://inis.iaea.org/records/16frp-vsh57
- Nasreen SZA, Mahjabeen N, Shahreen S. Postmenopausal bleeding: An update. Eur J Med Health Sci [Internet]. 2021;3(1):28–33. Available from: https://ej-med.org/index.php/ejmed/article/view/652
- Bukhari NA, Qazi WA, Jahan S, Asmat S, Akhter N, Jabeen R. Risk factors associated with postmenopausal bleeding and endometrial carcinoma. Prof Med J [Internet]. 2021;28(2):208–13. Available from: https://doi.org/10.29309/TPMJ/2021.28.02.3447
- Ramezanali F, Khalili G, Arabipoor A, Lankarani NB, Moini A. Relationships between serum luteinizing hormone level, endometrial thickness and body mass index in polycystic ovary syndrome patients with and without endometrial hyperplasia. Int J Fertil Steril [Internet]. 2016;10(1):36–41. Available from: https://doi.org/10.22074/ijfs.2016.4766
- Okman-Kilic T, Kucuk M. The effects of antihypertensive agents on endometrial thickness in asymptomatic, hypertensive, postmenopausal women. Menopause [Internet]. 2003;10(4):362–5. Available from: https://doi.org/10.1097/01.gme.0000051508.69832.ba
- Bornstein J, Auslender R, Goldstein S, Kohan R, Stolar Z, Abramovici H. Increased endometrial thickness in women with hypertension. Am J Obstet Gynecol [Internet]. 2000;183(3):583–7. Available from: https://doi.org/10.1067/mob.2000.106719
- Fatima SS, Naib JM, Sharafat Z, Mazhar T. Postmenopausal bleeding-an alarming symptom of endometrial carcinoma. J Med Sci. 2014;22(4):166–70. Available from: https://jmedsci.com/index.php/Jmedsci/article/view/249
- Wang L, Quan S, Bai E, Yang X. Analysis of clinical data of different endometrial pathological types in perimenopausal women with abnormal uterine bleeding. Front Oncol. 2024;14:1370681. Available from: https://doi.org/10.3389/fonc.2024.1370681
- Gök S, Atigan A, Gök BC. A new method that facilitates the diagnosis of endometrial cancer: is the ratio of endometrial thickness to the full thickness of the uterine wall and subcutaneous adipose tissue measurements. Menopause Rev. 2024;23(1):25–30. Available from: https://doi.org/10.5114/pm.2024.136961
- Ahmed AAM, Mohamed BEE, Abdel Moneim OAA. Comparative Study between the Diagnostic Accuracy of 3D Ultrasound and Hysteroscopy in Patient with Post Menposal Uterine Bleeding. Al-Azhar Int Med J. 2024;5(4):52. Available from: https://aimj.researchcommons.org/journal/vol5/iss4/52/
- Al-Kamil R. Clinical effectiveness of hysteroscopy in abnormal uterine bleeding. J Obstet Gynaecol. 2001;21(6):614-6. Available from: https://doi.org/10.1080/01443610120085609
- Pacheco JC, Kempers RD. Etiology of postmenopausal bleeding. Obstet Gynecol. 1968;32(1):40-46. Available from: https://pubmed.ncbi.nlm.nih.gov/5742088/
- Bettocchi S, Ceci O, Nappi L, Di Venere R, Masciopinto V, Pansini V, et al. Operative office hysteroscopy without anesthesia: analysis of 4863 cases performed with mechanical instruments. J Am Assoc Gynecol Laparosc. 2004;11(1):59–61. Available from: https://doi.org/10.1016/s1074-3804(05)60012-6
- Begum J, Samal R. A clinicopathological evaluation of postmenopausal bleeding and its correlation with risk factors for developing endometrial hyperplasia and cancer: a hospital-based prospective study. J Midlife Health. 2019;10(4):179–83. Available from: https://doi.org/10.4103/jmh.jmh_136_18
- Ghoubara A, Price MJ, Fahmy MSED, Ait-Allah AS, Ewies A. Prevalence of hyperplasia and cancer in endometrial polyps in women with postmenopausal bleeding: A systematic review and meta-analysis. Post Reprod Health. 2019;25(2):86–94. Available from: https://doi.org/10.1177/2053369119833583
- Tian Y, Bai B, Wang L, Zhou Z, Tang J. Contributing factors related to abnormal uterine bleeding in perimenopausal women: a case-control study. J Health Popul Nutr. 2024;43(1):52. Available from: https://jhpn.biomedcentral.com/articles/10.1186/s41043-024-00540-4
- Tahseen H, Khokhar S, Atif N, Qurban S, Khurshid HN, Maqsud M. Histopathological Diagnosis of Hysterectomy Specimens in Abnormal Uterine Bleeding. Pak J Med Health Sci. 2023;17(04):232. Available from: https://doi.org/10.53350/pjmhs2023174232