More Information
Submitted: March 05, 2024 | Approved: March 25, 2024 | Published: March 26, 2024
How to cite this article: Karami MH, Abdouss M.A General Evaluation of the Cellular Role in Drug Release: A Clinical Review Study. Clin J Obstet Gynecol. 2024; 7: 042-050.
DOI: 10.29328/journal.cjog.1001162
Copyright License: © 2024 Karami MH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Drug delivery; Nanoparticles; Cell-based drug delivery; Cell trafficking; Cell homing
A General Evaluation of the Cellular Role in Drug Release: A Clinical Review Study
Mohammad Hossein Karami* and Majid Abdouss*
Department of Chemistry, Amirkabir University of Technology, P.O. Box 15875-4413, Tehran, Iran
*Address for Correspondence: Mohammad Hossein Karami, Department of Chemistry, Amirkabir University of Technology, P.O. Box 15875-4413, Tehran, Iran,
Email: [email protected]
Majid Abdouss, Department of Chemistry, Amirkabir University of Technology, P.O. Box 15875-4413, Tehran, Iran
Cells have emerged as highly promising vehicles for delivering drugs due to their unique advantages. They have the ability to bypass immune recognition, navigate biological barriers, and reach difficult-to-access tissues through sensing and active movement. Over the past couple of decades, extensive research has been conducted to understand how cell carriers can overcome biological barriers and influence drug effectiveness. This has resulted in the development of engineered cells for targeted drug delivery to specific tissues. Despite the presence of exciting developments, a comprehensive understanding of the challenges and potential strategies is necessary for the effective clinical application of cell-based drug carriers. This review provides an overview of recent progress and novel concepts in cell-based drug carriers, as well as their potential for translation into clinical practice. Additionally, we delve into important factors and emerging strategies for designing the next generation of cell-based delivery technologies, with a particular emphasis on achieving greater accuracy and targeted drug administration.
Drug delivery systems (DDSs) play a vital role in optimizing drug efficacy and safety by efficiently transporting medications within the body. These systems are designed to overcome challenges such as drug degradation, poor solubility, limited bioavailability, and off-target effects while maximizing drug release, distribution, and targeting [1]. Utilizing carriers like nanoparticles, liposomes, or cell-based carriers enables precise drug delivery, reducing side effects and improving therapeutic outcomes. The development of DDS is crucial for advancing drug delivery and realizing the full potential of various medications in treating diseases [2]. Nanoparticles (NPs) have emerged as a promising approach to overcome biological barriers in drug delivery. Understanding the impact of NP properties on targeting capabilities allows for the customization of NPs for precise and targeted therapeutic delivery. Manipulating NP properties enhances their ability to reach specific cells or tissues, thus enhancing the effectiveness of drug delivery systems. While significant progress has been made, intravascular therapies still face challenges in precise targeting due to the complex nature of the body and rapid immune system elimination [3-5]. Cells have also been explored as drug carriers, with various techniques developed to utilize different cell types for targeting specific tissues. Overcoming obstacles such as complement-mediated attacks on modified cell carriers and immune rejection of allogeneic cell-based carriers are critical steps. Strategies involving cells with inherent transmigration capabilities, genetic modification to enhance the crossing of the blood-brain barrier, and engineered cell carriers with ECM-degrading enzymes are explored [6].
Recently, there have been significant advancements in the understanding of colon cancer treatment and prevention. The new classification system considers factors such as tumor size, histopathology, gene expression, DNA methylation, and oncogenic pathways [7-10]. The Cancer Genome Atlas (TCGA) and the Asian Cancer Research Group (ACRG) have proposed personalized approaches to treating colon cancer based on these classifications. Various studies have highlighted how different molecular groups can impact demographic and disease characteristics like age, tumor location, invasiveness, and stage [12-16]. Projects like the High-Tech Omics-Based Patient Evaluation Project (HOPE) have developed molecular markers to predict survival outcomes for patients undergoing radical gastrectomy [17]. Despite improvements in surgical techniques, research participation, and perioperative care, outcomes for colon cancer patients still vary among physicians and countries. To address these disparities and ensure quality care, volume-based referrals have been recommended as a reliable indicator of effective cancer treatment. The concept of "operational volume" introduced in 1979 by Luft HS, et al. emphasizes that high-volume hospitals tend to have better outcomes for complex surgical procedures [18-25].
The recent development of non-thermal physical plasma for medical objectives, along with its potential combination with Nanocarriers, is generating a lot of interest and excitement in the scientific community [24]. Researchers are optimistic about the possibilities this technology holds for innovative medical treatments and targeted drug delivery. The synergy between non-thermal physical plasma and Nanocarriers could open up new avenues for more effective and precise therapies in the future [25].
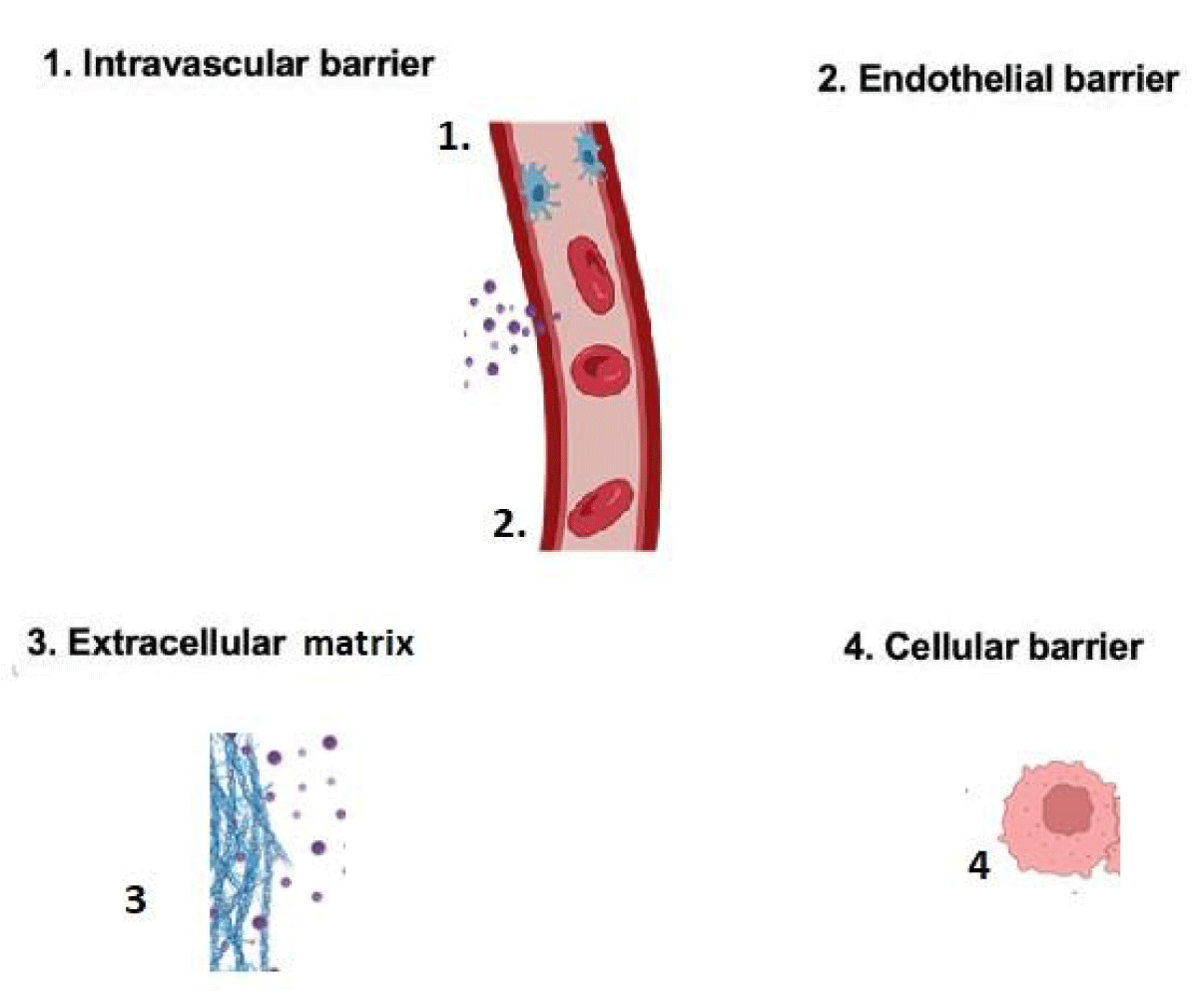
The review emphasizes the potential of cell-based drug delivery technologies to overcome biological barriers, highlighting the importance of genetic modification and engineering approaches. Innovative strategies to overcome barriers like the blood-brain barrier and extracellular matrix are essential for advancing cell-based drug delivery and improving treatment outcomes. The Figure 1 provides an overview of the intravascular administration
Figure 1: Presents an overview of the administered intravascularly.
In the past few years, there have been notable advancements in the field of engineering cells for precise drug delivery. This field focuses on improving the effectiveness and specificity of drug delivery while minimizing unwanted side effects. Researchers have developed innovative cell-based carriers, such as genetically modified T cells or stem cells, which can be engineered to express specific receptors or enzymes for targeted drug delivery [7]. Additionally, nanotechnology has enabled the development of engineered nanoparticles that can be loaded with drugs for precise delivery to specific cells or tissues. Nevertheless, there are still several challenges that need to be addressed in order to successfully translate these advancements into clinical settings. It is important to carefully address and resolve concerns related to the source of cells, manufacturing costs, and the fate of the cell carriers within the body [8]. These factors must be thoroughly considered to ensure the successful application of cell-based drug delivery techniques. Combining cell therapy with cell-mediated drug delivery is an encouraging strategy that aims to enhance therapeutic effects. The field of engineering cells for precision drug delivery shows great potential in revolutionizing drug delivery methods through ongoing research and interdisciplinary collaboration. This review explores recent advancements, clinical applications, and the potential of cell-based drug delivery technologies to improve treatment outcomes [9-11].
Recent advancements in the cell-based drug carriers
This review offers a comprehensive overview of recent advancements in cell-mediated drug delivery technologies. It categorizes these advancements based on specific cell types and their respective applications, as presented in Table 1. The discussion highlights the innovative concepts and breakthroughs in this field, demonstrating the wide range of possibilities and potential of cell-mediated delivery systems [12-16]. By harnessing the unique characteristics of different cell types, researchers have developed sophisticated drug delivery approaches that offer precise and targeted therapeutic delivery. This review serves as a valuable resource for understanding the current status and future directions of cell-mediated drug delivery, offering insights into the progress achieved and the potential impact of these technologies in enhancing therapeutic outcomes [17].
| Table 1: Categorization of Advancements in Cell-Based Drug Delivery Technologies. | ||
| Cell carrier | Loading( method) | Ref |
| RBC | Hitchhiking Antibody coupling Genetic engineering |
[12] |
| T cell | Biomimetic materials Antibody coupling |
[14] |
| Platelet | Ligand affinity Ligand affinity |
[15] |
| Stem cell | Conjugation | [16] |
| Monocyte and macrophages |
Cellular uptake | [17] |
| Neutrophil | Antibody coupling | [11] |
| Other cells | Genetic Loading Click chemistry |
[10] |
Red blood cells
Red blood cells (RBCs) have gained significant attention as potential carriers for drug delivery due to their unique characteristics. Recent advancements in RBC-based drug delivery have focused on loading drugs inside RBCs or attaching them to the RBC surface while preserving the RBCs' normal functions. One exciting development is the concept of stimuli-responsive RBCs, which can release drugs in response to specific triggers, either from within the RBCs or from external stimuli [18]. Another promising approach is RBC hitchhiking with nanoparticles, where RBCs are used to transport nanoparticles loaded with drugs to specific target organs or tissues. These innovative strategies have shown promise in enhancing drug delivery for various conditions, such as lung metastasis and autoimmune diseases. Additionally, RBCs possess the ability to induce antigen-specific tolerance, which opens up potential applications in reducing the production of antibodies against therapeutic drugs. These recent advancements demonstrate the potential of RBCs in overcoming challenges and improving the efficacy of cell-mediated drug delivery [19-21].
Leukocytes: Leukocytes, which encompass neutrophils, monocytes, macrophages, and T cells, play vital roles in immune defense, blood clotting, and tissue healing. Scientists have acknowledged the potential of harnessing these cells' inherent homing capabilities for drug delivery purposes [22]. By engineering these cells, researchers have devised strategies to utilize them as carriers for transporting therapeutic agents to specific locations within the body. When there is inflammation, infection, or cancer, leukocytes are naturally drawn to the affected sites. By modifying these cells, they can be guided to deliver drugs precisely to the desired locations. This innovative approach capitalizes on the natural migration and targeting abilities of leukocytes, presenting a promising avenue for targeted drug delivery and improved therapeutic outcomes [23].
Neutrophils: Neutrophils, the most abundant leukocyte population, possess a pivotal role in combating infections as the initial line of defense [24]. Their impressive capacity to be attracted to sites of inflammation such as the blood-brain barrier has fascinated researchers in the field of drug delivery. Capitalizing on these unique attributes, scientists have successfully employed neutrophils as carriers for targeted drug delivery. This innovative approach holds immense promise in the treatment of inflammatory brain and lung diseases, as well as cancer [25]. Strategies such as loading drugs directly into neutrophils or utilizing nanoparticles that interact with specific receptors on these cells have been explored to augment drug delivery to inflamed tissues. Furthermore, investigations into manipulating the tumor microenvironment to enhance neutrophil recruitment have been undertaken to improve drug delivery in cancer therapy [26]. Another effective tactic involves encapsulating drugs within liposomes that are then loaded into neutrophils, facilitating the precise delivery of therapeutics to inflammatory tissues. Overall, harnessing the inherent abilities of neutrophils as drug carriers presents exciting opportunities for targeted and efficient drug delivery across diverse disease contexts.
Monocytes and macrophages: Monocytes, which are a specific type of white blood cells, are generated in the bone marrow and circulate throughout the bloodstream [27]. Upon migrating to nearby tissues, they have the ability to differentiate into either macrophages or dendritic cells. Monocytes, a subset of leukocytes, possess chemokine receptors that guide them towards areas of inflammation. This natural behavior has been harnessed by researchers to use monocytes/macrophages as carriers for targeted drug delivery. By loading nanoparticles, such as nanoenzymes or indinavir NPs, into these cells, effective drug delivery to the brain has been achieved [28]. This approach shows promise for treating conditions like Parkinson's disease and inhibiting HIV replication. To simplify targeted drug delivery, an innovative strategy called in situ monocyte hitchhiking has been developed. This method takes advantage of the inherent migration of monocytes to inflamed tissues. By leveraging this migration, the need for complex cell isolation or manipulation is eliminated, streamlining the process and potentially improving efficiency [29-31].
T Cells: Differentiated subsets of T-cells, including helper T cells, cytotoxic T cells, and regulatory T cells, can be derived from bone marrow hematopoietic cells [32]. Recent research by Irvine, et al. has demonstrated the potential of utilizing T-cells as carriers for drug-loaded nanoparticles. By covalently attaching these nanoparticles to the surface of T-cells, targeted delivery of chemotherapeutics, particularly lymphoma, can be achieved. This innovative approach holds significant promise in improving cancer treatment outcomes by utilizing T-cells as vehicles for delivering therapeutic agents [33].
The study found that loading nanoparticles with immunomodulators and linking them to T-cells enhances the activity of these cells [34]. Furthermore, the researchers demonstrated that the release of drugs from the nanoparticles can be controlled to specifically target sites where T-cells encounter antigens, such as the tumor microenvironment. This targeted drug release strategy has the potential to enhance treatment effectiveness while minimizing adverse effects. In summary, the use of T-cell-targeting nanoparticles presents an exciting opportunity for in vivo T-cell engineering. This advancement allows for the full utilization of engineered T-cells. By directly delivering nanoparticles to specific locations within the body, this approach offers a more efficient and effective means of harnessing engineered T-cells for therapeutic purposes [35].
Platelets: Platelets, which are produced by megakaryocytes, have essential functions in blood clotting, inflammation, and the dissemination of cancer [36]. They possess inherent targeting capabilities that enable them to specifically home in on injured tissues, tumor sites, and areas of vascular inflammation. The unique attribute of platelets makes them highly valuable for targeted drug delivery to specific locations within the body [37]. The interaction between platelets and circulating tumor cells (CTCs) has emerged as a promising focus for preventing the spread and recurrence of cancer. Activated platelets can form aggregates with CTCs, providing a shield against immune system detection. Disrupting this interaction holds significant therapeutic potential for slowing down cancer growth and improving patient outcomes [38]. Moreover, platelets naturally possess the ability to target tumors and surgical wounds, making them ideal carriers for agents that block the PD-1/PD-L1 pathway. This pathway is responsible for inhibiting the immune response against tumors. However, the limited presence of immune cells surrounding the tumor presents obstacles to the effectiveness of immune checkpoint inhibitors. By utilizing platelets as delivery vehicles, it is possible to efficiently transport PD-1/PD-L1 blockade agents to tumor sites, potentially improving the effectiveness of these inhibitors [39].
Stem cells: Stem cells consist of a diverse group of cells capable of differentiating into specific cell types. One particular type is called mesenchymal stem cells (MSCs). MSCs are guided to inflammatory tissues, including tumors, by chemokine receptors like CXCR4, CXCR12, and CCR2 [40]. Enhancing drug delivery to tumors by loading MSCs with drugs or nanoparticles has shown promise in improving the effectiveness of cancer treatments for various types, such as lung, breast, glioma, and lymphoma. However, using MSCs as carriers presents challenges, including limited targeting capabilities and uncontrolled differentiation. Addressing these limitations, a novel technology called cardiocytes has been developed to enhance MSC targeting and provide better control over their differentiation, offering improved safety and efficacy for MSC-based therapies. The emergence of cargocyte technology holds potential for broader applications and advancements in cell-based therapies across diverse fields [41].
Other cells: Various cell types, including NK cells, DCs, adipocytes, and bacteria, have been modified for drug delivery purposes in addition to MSCs [42]. The selection of a specific cell type as a carrier depends on the desired objectives of drug delivery and the unique characteristics of the cells involved. For example, certain strains of bacteria have a natural tendency to accumulate in tumors, making them an appealing choice for targeted drug delivery using synthetic biology or biomaterials. Adipocytes, on the other hand, possess the ability to store hydrophobic drugs within lipid droplets, making them advantageous carriers for such drugs [43]. Numerous studies have investigated the use of adipocytes as biocompatible vehicles for delivering anticancer drugs. In summary, the field of cell-based drug delivery is highly diverse, with different cell types being engineered to meet specific therapeutic requirements and leverage their distinct biological properties. This research is important in the development of advanced drug delivery systems, with the potential to revolutionize healthcare and improve patient outcomes [44].
Clinical landscape of cell-based drug carriers
The rapid progress in the clinical field of cell-based drug carriers holds immense potential for the future of healthcare. A notable example of this is CAR-T cell therapy, which involves genetically modifying a patient's T cells to produce chimeric antigen receptors (CARs) that specifically target cancer cells [45]. This revolutionary method has the ability to revolutionize cancer treatment by enhancing the immune system's capacity to recognize and eliminate cancerous cells. CAR-T cell therapies like Kymriah and Yescarta have been approved for specific types of leukemia and lymphoma, demonstrating the success of this treatment approach. Another area that demonstrates significant potential is the utilization of mesenchymal stem cells (MSCs) in diverse clinical applications [46]. Prochymal, a therapy based on MSCs, has received approval in certain countries for the treatment of graft-versus-host disease (GVHD). Additionally, MSCs are being extensively studied for their regenerative properties in various conditions, including osteoarthritis, cardiovascular diseases, and inflammatory bowel disease [47]. These therapeutic applications highlight the potential of MSCs in promoting tissue repair and modulating the immune system. Clinical trials currently underway are actively investigating the utilization of other cell types for targeted drug delivery. This involves the exploration of various cell types, including dendritic cells, natural killer cells, and engineered immune cells, with the aim of developing innovative strategies to deliver drugs directly to specific cells or tissues [48]. These trials seek to enhance the precision and efficacy of DDSs, ultimately leading to improved outcomes for patients across a wide range of medical conditions. Natural killer (NK) cells, dendritic cells (DCs), and adipocytes are being studied for their unique characteristics that can enhance drug delivery efficacy and specificity in cancer treatment. The field of cell-based drug carriers is continuously evolving and expanding (Table 2).
| Table 2: Cell-based drug delivery technologies. | ||
| Cell carrier | Loading | Ref |
| RBC | Microfluidic-based cell squeeze Red Cell Loader Genetic engineering Ex vivo loading Hypotonic dialysis |
[10] |
| NK cell | Genetic engineering | [10] |
| MSCs | Genetic engineering -Stem Cells Expressing TRAIL MSCs infected with an oncolytic measles virus encoding NIS |
[10] |
| T cell | T cells with IL-15Fc- Genetic engineering | [10] |
Some therapies have already made notable progress, while others are still in the initial phases of development. Further research and clinical trials will continue to advance the field, bringing us closer to the widespread use of cell-based drug carriers in various therapeutic areas, revolutionizing the approach to disease treatment [43].
Cell-based drug carriers are showing great promise in the clinical landscape, with several therapies advancing to clinical trials or receiving regulatory approval. One prominent illustration is CAR-T cell therapy, which entails the alteration of a patient's T cells to incorporate chimeric antigen receptors (CARs) capable of targeting cancer cells [49].
Besides MSCs, scientists are also focusing on engineering other cell types like NK cells, DCs, and adipocytes for the purpose of targeted drug delivery in cancer treatment, in addition to CAR-T cells. These cell-based carriers offer unique properties that can enhance drug delivery efficacy and specificity [50,51]. Some therapies in the field of cell-based drug carriers have achieved significant progress in the clinical landscape, while others are still in the initial stages of development, indicating a dynamic and expanding field. Continued research and clinical trials will further advance the field and bring us closer to the widespread use of cell-based drug carriers in various therapeutic areas. Overall, cell-based drug carriers hold immense potential for revolutionizing medicine by providing more precise and effective treatments for various diseases. The ongoing progress in this field brings hope for improved patient outcomes and a brighter future in healthcare [52].
Controllable payload-carrier cell association
Efficiently loading therapeutic payloads onto carrier cells is a crucial step in developing effective cell-based drug delivery systems. There are various techniques available for loading drugs or drug nanoparticles (NPs) onto cells [53]. There are several methods available for loading drugs into cells, including phagocytosis-mediated uptake, and cationic polymers. These techniques enable drugs or NPs to be taken up inside the cell for subsequent release and delivery. Surface loading involves attaching drugs to the cell membrane, protecting them from intracellular conditions, and allowing controlled release [49]. To load drugs into cells, different methods can be employed, including lipid insertion, or specific biological interactions (non-covalent nanoparticle hitchhiking, chemical conjugation). These techniques enable efficient delivery of drugs into cells, allowing for targeted and effective treatment. Polymer coatings, electrostatic interactions, and metal-assisted self-assembly can also be used to modify the cell surface for drug loading. Genetic engineering is a process that involves modifying cells to produce targeted proteins or ligands. This can be achieved by introducing or altering genes in the cells. The modified cells are then capable of producing the desired molecules, which can be located on the cell surface (secreted outside or inside the cells). This allows precise control over protein localization and has been successfully applied to various cell types for treating diseases like cancer and inflammation [47].
In summary, loading therapeutic payloads onto carrier cells is a critical aspect of developing effective cell-based drug delivery systems. Different methods, such as intracellular loading, surface loading, and genetic engineering, offer various approaches for achieving efficient drug delivery [54].
Modulation of the target tissue environment
To effectively target disease tissues, cell carriers rely on chemokine secretion and chemokine-chemokine receptor interactions to guide their migration. Modifying the surrounding tissue environment to optimize the attraction of cell carriers has emerged as a promising approach. Implementing the infusion of chemotactic agents into the desired tissues creates a chemokine gradient, thereby amplifying the recruitment of cell carriers equipped with chemokine receptors [55]. This helps direct the cell carriers towards the desired location. Furthermore, the utilization of cell-recruiting agents, such as ligands or small molecules, can enhance the recruitment and engraftment of cell-based carriers within the specific tissue of interest. These cell-engagers facilitate the interaction between the carriers and the tissue, enhancing their ability to deliver therapeutic payloads. Additionally, various physiological or pharmacological stimuli, such as sensitizing agents, ionizing radiation, or oxygen deficiency, can be harnessed to elicit an inflammatory response in the targeted tissue [56]. This leads to an augmented release of chemokines, thereby promoting the infiltration of cell carriers into the tissue. Modifying the milieu of the intended tissue provides fresh avenues for bolstering the capabilities of cell-based conveyors. It is a more viable strategy when contrasted with altering the conveyors themselves. By optimizing the environment of the target tissue, researchers can enhance the attraction and persistence of cell conveyors, ultimately amplifying the efficiency of cell-based pharmaceutical delivery systems. In a nutshell, successful precision targeting of afflicted tissues necessitates utilizing chemokine signaling and interactions, alongside manipulating the surroundings of the desired tissue to amplify the potential of cell-based transporters. Ongoing research in this field will continue to advance the development of effective cell-based drug delivery strategies [57].
Trafficking capability of carrier cells
The efficacy of cell-based medication delivery relies on the capacity of carrier cells to navigate through the body and selectively target specific tissues. However, the trafficking ability of cell-based carriers often falls short, resulting in limited targeting and unintended accumulation. To address this challenge, it is essential to consider cellular dynamics and choose the most suitable cell carrier for a given ailment. For instance, macrophages exhibit phagocytic properties, while dendritic cells are known for their antigen-presenting capabilities [58]. Even within a single cell type, different subsets may display distinct tissue navigation abilities. To enhance the trafficking capability of cell carriers, various strategies have been employed. One approach entails modulating the expression of chemokine receptors and adhesion molecules, which has demonstrated success in improving the targeting of cell carriers such as mesenchymal stem cells, natural killer cells, macrophages, and T cells [59]. Additionally, the administration route of cell-based carriers plays a critical role in achieving optimal targeting. For example, intravenous administration of cell carriers often necessitates overcoming the lung entrapment phenomenon before reaching the intended target tissues. By comprehending and optimizing these factors, both native and engineered cell carriers can be effectively utilized to enhance the precision and efficiency of drug delivery. Ongoing research in this field aims to further unlock the potential of cell-based carriers for targeted drug delivery in various disease conditions [55].
Biological behavior and activity of cell carriers
The biophysical characteristics of cellular carriers, such as their phenotype, migratory capacity, and activation state, play a pivotal role in determining the success of drug delivery. While previous investigations have primarily focused on minimizing the impact of drug loading on cellular trafficking, there is an increasing demand to explore strategies for modulating the biophysical behavior of cellular carriers to enhance drug delivery outcomes [17-23]. For instance, by priming macrophages with polarizing agents, it is possible to optimize their behavior and improve delivery efficiency. Similarly, manipulating the activation state of neutrophils and platelets can augment their delivery capabilities. Tailoring the biophysical behavior of cellular carriers is crucial in the advancement of more effective cell-based drug delivery technologies [24].
Traditionally, cell-mediated drug delivery has centered on utilizing cells solely as carriers to improve delivery efficiency. However, it is now evident that many cell types possess distinct intrinsic biological functions beyond their role as carriers [28]. For example, antigen-specific CD8 T cells exhibit cytotoxic activity, while Tregs have the ability to regulate T cell responses. To harness these functions, researchers have developed a technique known as "cellular backpacks." These backpacks are pliable, disc-shaped microparticles that can be attached to the surface of macrophages and T cells, enabling control over their phenotype and enhancing therapeutic efficacy. By attaching backpacks to macrophages, drug delivery to inflamed lungs and tumors can be optimized. Similarly, attaching backpacks to T cells enables efficient drug delivery to target tissues and enhances their activity for improved therapeutic outcomes [55]. For instance, TCR-signaling-responsive cytokine nanogels attached to the surface of antigen-specific T cells can release cytokines like IL-15 in response to encounters with antigens, thereby boosting their cytotoxic activity. Additionally, attaching TCR-signaling-responsive IL-2 nanogels to the surface of Tregs can enhance their activity and suppress alloimmunity. These backpack technologies present a promising approach for targeted therapeutic delivery and enhancing the effectiveness of T cell-based therapies by leveraging the unique biophysical functions of specific cell types [56].
Cellular hitchhiking
The ex-situ approach to cell-based drug delivery involves isolating carrier cells, loading them with drugs outside the body, and subsequently reintroducing them into patients. The selection of carrier cell source has a significant impact on the manufacturing process in this approach [57]. Allogeneic cells can be utilized as a readily available resource, but concerns regarding immune rejection may arise. Conversely, autologous cells necessitate fresh isolation and manufacturing just before administration, which may not be suitable for acute diseases with time constraints. In contrast, the in situ cellular hitchhiking approach is a more recent strategy that eliminates the need for isolating carrier cells [58]. It entails the development of nanoparticles (NPs) with surface modifications that can bind to specific carrier cells within the body upon intravenous administration. This approach has the potential to reduce manufacturing time and costs compared to the ex-situ approach. Recent advancements in NP barcoding and other technologies can facilitate the screening of NPs for in situ hitchhiking [59].
One approach to developing in situ cellular hitchhiking NPs involves the conjugation of targeting ligands onto their surface to recognize specific blood cell types. Non-targeted NPs with suitable physicochemical properties can also bind to specific cell types in vivo [55]. However, challenges exist in designing NPs that efficiently hitchhike on specific cells and understanding the underlying engineering principles. Further research is needed to identify surface markers that are exclusively expressed on target cells and to comprehend the potential uptake of NPs by off-target cells. Additionally, controlling the localization of NPs within target cells and achieving controlled release of drugs/NPs from carrier cells remain ongoing challenges in the in situ cellular hitchhiking approach [59-61].
The field of cell-based drug delivery has experienced significant advances, offering promising solutions to overcome the limitations of synthetic material-based carriers. Living carriers, with their unique biological capabilities, have proven to be well-suited for addressing challenges faced by conventional delivery systems. Recent studies have revealed the superior efficiency of certain cell-based carriers, such as neutrophils and monocytes, in drug delivery, particularly in crossing the challenging blood-brain barrier. However, there are still several areas that require further exploration. Research efforts should focus on understanding the migration patterns of specific cells in different diseases, their impact on disease progression, and their ability to target specific tissues. From an engineering perspective, optimizing cell types, achieving precise drug loading within carrier cells, controlling drug release, and enhancing tissue targeting pose significant challenges. Additionally, factors like cell source, availability, manufacturing costs, and the fate of cell carriers within the body need to be carefully considered for successful translation to clinical applications. The synergy of multidisciplinary collaboration will be crucial in filling these knowledge gaps and propelling the advancement of more efficient cell-based drug delivery technologies. The convergence of cell therapy and cell-mediated drug delivery holds immense potential for achieving enhanced therapeutic outcomes in the future. By combining the expertise of professionals from diverse fields, such as biotechnology, pharmacology, immunology, and biomaterials, we can harness their collective knowledge to address the challenges and drive the development of novel and effective cell-based drug delivery strategies.
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contributions statement
Mohammad Hossein Karami: Supervision, Formal analysis, Data Curation Investigation, Resources, Writing – Original Draft, Methodology, Conceptualization, Validation, Resources, Writing- Review & Editing, Visualization, Project administration, Funding acquisition, Methodology. Majid Abdouss: Validation.
- Tewabe A, Abate A, Tamrie M, Seyfu A, Abdela Siraj E. Targeted Drug Delivery - From Magic Bullet to Nanomedicine: Principles, Challenges, and Future Perspectives. J Multidiscip Healthc. 2021 Jul 5;14:1711-1724. doi: 10.2147/JMDH.S313968. PMID: 34267523; PMCID: PMC8275483.
- Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015 Sep;33(9):941-51. doi: 10.1038/nbt.3330. PMID: 26348965; PMCID: PMC4978509.
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;
- Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. 2016 Jun 3;1(1):10-29. doi: 10.1002/btm2.10003. PMID: 29313004; PMCID: PMC5689513.
- Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011 Mar 18;63(3):131-5. doi: 10.1016/j.addr.2010.03.011. Epub 2010 Mar 18. PMID: 20304019.
- Zhao Z, Ukidve A, Krishnan V, Mitragotri S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv Drug Deliv Rev. 2019 Mar 15;143:3-21. doi: 10.1016/j.addr.2019.01.002. Epub 2019 Jan 11. PMID: 30639257.
- Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011 Jul 5;108(27):10980-5. doi: 10.1073/pnas.1106634108. Epub 2011 Jun 20. PMID: 21690347; PMCID: PMC3131364.
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008 Dec;8(12):958-69. doi: 10.1038/nri2448. Erratum in: Nat Rev Immunol.2010 Jun;10(6):460. PMID: 19029990; PMCID: PMC2724991.
- Zhao Z, Ukidve A, Kim J, Mitragotri S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell. 2020 Apr 2;181(1):151-167. doi: 10.1016/j.cell.2020.02.001. PMID: 32243788.
- Li ZT, Wang YX, Ding YY, Repp L, Kwon GS, Hu QY. Cell-Based Delivery Systems: Emerging Carriers for Immunotherapy, Adv. Funct. Mater. 2021; 31.
- Marano L, Verre L, Carbone L, Poto GE, Fusario D, Venezia DF, Calomino N, Kaźmierczak-Siedlecka K, Polom K, Marrelli D, Roviello F, Kok JHH, Vashist Y. Current Trends in Volume and Surgical Outcomes in Gastric Cancer. J Clin Med. 2023 Apr 4;12(7):2708. doi: 10.3390/jcm12072708. PMID: 37048791; PMCID: PMC10094776.
- Yang L, Yang Y, Chen Y, Xu Y, Peng J. Cell-based drug delivery systems and their in vivo fate. Adv Drug Deliv Rev. 2022 Aug;187:114394. doi: 10.1016/j.addr.2022.114394. Epub 2022 Jun 17. PMID: 35718252.
- Glassman PM, Hood ED, Ferguson LT, Zhao Z, Siegel DL, Mitragotri S, Brenner JS, Muzykantov VR. Red blood cells: The metamorphosis of a neglected carrier into the natural mothership for artificial nanocarriers. Adv Drug Deliv Rev. 2021 Nov;178:113992. doi: 10.1016/j.addr.2021.113992. Epub 2021 Sep 29. PMID: 34597748; PMCID: PMC8556370.
- Brenner JS, Mitragotri S, Muzykantov VR. Red Blood Cell Hitchhiking: A Novel Approach for Vascular Delivery of Nanocarriers. Annu Rev Biomed Eng. 2021 Jul 13;23:225-248. doi: 10.1146/annurev-bioeng-121219-024239. Epub 2021 Mar 31. PMID: 33788581; PMCID: PMC8277719.
- Qasim W, Zhan H, Samarasinghe S, Adams S, Amrolia P, Stafford S, Butler K, Rivat C, Wright G, Somana K, Ghorashian S, Pinner D, Ahsan G, Gilmour K, Lucchini G, Inglott S, Mifsud W, Chiesa R, Peggs KS, Chan L, Farzeneh F, Thrasher AJ, Vora A, Pule M, Veys P. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017 Jan 25;9(374):eaaj2013. doi: 10.1126/scitranslmed.aaj2013. Erratum in: Sci Transl Med. 2017 Feb 15;9(377):null. PMID: 28123068.
- Gravina A, Tediashvili G, Rajalingam R, Quandt Z, Deisenroth C, Schrepfer S, Deuse T. Protection of cell therapeutics from antibody-mediated killing by CD64 overexpression, Nature. Biotechnology.
- Pandey PK, Sharma AK, Gupta U. Blood brain barrier: An overview on strategies in drug delivery, realistic in vitro modeling and in vivo live tracking. Tissue Barriers. 2015 Dec 15;4(1):e1129476. doi: 10.1080/21688370.2015.1129476. PMID: 27141418; PMCID: PMC4836458.
- Afergan E, Epstein H, Dahan R, Koroukhov N, Rohekar K, Danenberg HD, Golomb G. Delivery of serotonin to the brain by monocytes following phagocytosis of liposomes. J Control Release. 2008 Dec 8;132(2):84-90. doi: 10.1016/j.jconrel.2008.08.017. Epub 2008 Sep 4. PMID: 18805446.
- Karami MH, Abdouss M, Rahdar A, Pandey A. Graphene quantum dots: background, synthesis methods, and applications as nanocarrier in drug delivery and cancer treatment: an updated review. Inorg Chem Commun. 2024; 161:112032.
- Bhan A, Ansari K, Chen MY, Jandial R. Human induced pluripotent stem cell-derived platelets loaded with lapatinib effectively target HER2+ breast cancer metastasis to the brain. Sci Rep. 2021 Oct 15;11(1):16866. doi: 10.1038/s41598-021-96351-2. Retraction in: Sci Rep. 2024 Mar 12;14(1):5972. PMID: 34654856; PMCID: PMC8521584.
- Kremer V, Ligtenberg MA, Zendehdel R, Seitz C, Duivenvoorden A, Wennerberg E, Colón E, Scherman-Plogell AH, Lundqvist A. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J Immunother Cancer. 2017 Sep 19;5(1):73. doi: 10.1186/s40425-017-0275-9. Erratum in: J Immunother Cancer. 2017 Nov 6;5(1):88. PMID: 28923105; PMCID: PMC5604543.
- Hamadani CM, Goetz MJ, Mitragotri S, Tanner EEL. Protein-avoidant ionic liquid (PAIL)-coated nanoparticles to increase bloodstream circulation and drive biodistribution. Sci Adv. 2020 Nov 25;6(48):eabd7563. doi: 10.1126/sciadv.abd7563. PMID: 33239302; PMCID: PMC7688330.
- Blagovic K, Smith CK, Ramakrishnan A, Moore L, Soto DR, Thompson Z, Stockmann AP, Kruszelnicki S, Thakkar A, Murray J, Torres S, Wondimagegnhu B, Yi R, Dadgar M, Paracha AM, Page C, Clear L, Chaudhry OA, Myint M, Bridgen DT, Gilbert JB, Seidl KJ, Sharei A, Loughhead S, Bernstein H, Yarar D. Engineered red blood cells (activating antigen carriers) drive potent T cell responses and tumor regression in mice. Front Immunol. 2022 Oct 3;13:1015585. doi: 10.3389/fimmu.2022.1015585. PMID: 36263022; PMCID: PMC9573954.
- Huang J, Zhang L, Wan D, Zhou L, Zheng S, Lin S, Qiao Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct Target Ther. 2021 Apr 23;6(1):153. doi: 10.1038/s41392-021-00544-0. PMID: 33888679; PMCID: PMC8062524.
- Karami MH, Pourmadadi M, Abdouss M, Kalaee MR, Moradi O, Rahdar A, Díez-Pascual AM. Novel chitosan/γ-alumina/carbon quantum dot hydrogel nanocarrier for targeted drug delivery. Int J Biol Macromol. 2023 Aug 15;251:126280. doi: 10.1016/j.ijbiomac.2023.126280. Epub ahead of print. PMID: 37591420.
- Kakarla S, Chow KK, Mata M, Shaffer DR, Song XT, Wu MF, Liu H, Wang LL, Rowley DR, Pfizenmaier K, Gottschalk S. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther. 2013 Aug;21(8):1611-20. doi: 10.1038/mt.2013.110. Epub 2013 Jun 4. PMID: 23732988; PMCID: PMC3734659.
- Zhang W, Liu L, Su H, Liu Q, Shen J, Dai H, Zheng W, Lu Y, Zhang W, Bei Y, Shen P. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br J Cancer. 2019 Nov;121(10):837-845. doi: 10.1038/s41416-019-0578-3. Epub 2019 Oct 1. PMID: 31570753; PMCID: PMC6889154.
- Chu D, Dong X, Zhao Q, Gu J, Wang Z. Photosensitization Priming of Tumor Microenvironments Improves Delivery of Nanotherapeutics via Neutrophil Infiltration. Adv Mater. 2017 Jul;29(27):10.1002/adma.201701021. doi: 10.1002/adma.201701021. Epub 2017 May 15. PMID: 28504320; PMCID: PMC5510494.
- Shields CW 4th, Evans MA, Wang LL, Baugh N, Iyer S, Wu D, Zhao Z, Pusuluri A, Ukidve A, Pan DC, Mitragotri S. Cellular backpacks for macrophage immunotherapy. Sci Adv. 2020 Apr 29;6(18):eaaz6579. doi: 10.1126/sciadv.aaz6579. PMID: 32494680; PMCID: PMC7190308.
- Karami MH, Abdouss M. Assessing Particle Size and Surface Charge in Drug Carrier Nanoparticles for Enhanced Cancer Treatment: A Comprehensive Review Utilizing DLS and Zeta Potential Characterization. PSPRJ. 2024; 5(3): 000615.
- Karami MH, Abdouss M, Karami M. Evaluation of in vitro and ex vivo models for studying the effectiveness of vaginal drug systems in controlling microbe infections: A systematic review. Clin J Obst Gynecol. 2023; 6: 201-215.
- Li J, Ding Y, Cheng Q, Gao C, Wei J, Wang Z, Huang Q, Wang R. Supramolecular erythrocytes-hitchhiking drug delivery system for specific therapy of acute pneumonia. J Control Release. 2022 Oct;350:777-786. doi: 10.1016/j.jconrel.2022.08.029. Epub 2022 Sep 12. PMID: 35995300.
- Han X, Wang C, Liu Z. Red Blood Cells as Smart Delivery Systems. Bioconjug Chem. 2018 Apr 18;29(4):852-860. doi: 10.1021/acs.bioconjchem.7b00758. Epub 2018 Jan 22. PMID: 29298380.
- Zhang E, Phan P, Algarni HA, Zhao Z. Red Blood Cell Inspired Strategies for Drug Delivery: Emerging Concepts and New Advances. Pharm Res. 2022 Nov;39(11):2673-2698. doi: 10.1007/s11095-022-03328-5. Epub 2022 Jul 7. PMID: 35794397.
- Karami MH, Abdouss M. Recent advances of carbon quantum dots in tumor imaging. Nanomed J. 2024; 11(1): 13-35.
- Ihler GM, Glew RH, Schnure FW. Enzyme loading of erythrocytes. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2663-6. doi: 10.1073/pnas.70.9.2663. PMID: 4354859; PMCID: PMC427078.
- Li Y, Raza F, Liu Y, Wei Y, Rong R, Zheng M, Yuan W, Su J, Qiu M, Li Y, Raza F, Liu Y, Wei Y, Rong R, Zheng M, Yuan W, Su J, Qiu M. Clinical progress and advanced research of red blood cells based drug delivery system. Biomaterials. 2021 Dec;279:121202. doi: 10.1016/j.biomaterials.2021.121202. Epub 2021 Oct 22. PMID: 34749072.
- Rossi L, Pierigè F, Aliano MP, Magnani M. Ongoing Developments and Clinical Progress in Drug-Loaded Red Blood Cell Technologies. BioDrugs. 2020 Jun;34(3):265-272. doi: 10.1007/s40259-020-00415-0. PMID: 32198632; PMCID: PMC7211199.
- Su J, Sun H, Meng Q, Yin Q, Zhang P, Zhang Z, Yu H, Li Y. Bioinspired nanoparticles with NIR-controlled drug release for synergetic chemophotothermal therapy of metastatic breast cancer, Adv. Funct. Mater. 2016; 26: 7495-7506.
- Leuzzi V, Micheli R, D'Agnano D, Molinaro A, Venturi T, Plebani A, Soresina A, Marini M, Ferremi Leali P, Quinti I, Pietrogrande MC, Finocchi A, Fazzi E, Chessa L, Magnani M. Positive effect of erythrocyte-delivered dexamethasone in ataxia-telangiectasia. Neurol Neuroimmunol Neuroinflamm. 2015 Apr 9;2(3):e98. doi: 10.1212/NXI.0000000000000098. PMID: 25884015; PMCID: PMC4396528.
- Kontos S, Kourtis IC, Dane KY, Hubbell JA. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc Natl Acad Sci U S A. 2013 Jan 2;110(1):E60-8. doi: 10.1073/pnas.1216353110. Epub 2012 Dec 17. PMID: 23248266; PMCID: PMC3538192.
- Pishesha N, Bilate AM, Wibowo MC, Huang NJ, Li Z, Deshycka R, Bousbaine D, Li H, Patterson HC, Dougan SK, Maruyama T, Lodish HF, Ploegh HL. Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease. Proc Natl Acad Sci U S A. 2017 Mar 21;114(12):3157-3162. doi: 10.1073/pnas.1701746114. Epub 2017 Mar 7. Erratum in: Proc Natl Acad Sci U S A. 2017 Apr 25;114(17 ):E3583. Dhesycka, Rhogerry [corrected to Deshycka, Rhogerry]. PMID: 28270614; PMCID: PMC5373388.
- Zhao Z, Ukidve A, Gao Y, Kim J, Mitragotri S. Erythrocyte leveraged chemotherapy (ELeCt): Nanoparticle assembly on erythrocyte surface to combat lung metastasis. Sci Adv. 2019 Nov 13;5(11):eaax9250. doi: 10.1126/sciadv.aax9250. PMID: 31763454; PMCID: PMC6853768.
- Wang C, Ye Y, Sun W, Yu J, Wang J, Lawrence DS, Buse JB, Gu Z. Red Blood Cells for Glucose-Responsive Insulin Delivery. Adv Mater. 2017 May;29(18). doi: 10.1002/adma.201606617. Epub 2017 Mar 7. PMID: 28267235.
- Gao M, Hu A, Sun X, Wang C, Dong Z, Feng L, Liu Z. Photosensitizer Decorated Red Blood Cells as an Ultrasensitive Light-Responsive Drug Delivery System. ACS Appl Mater Interfaces. 2017 Feb 22;9(7):5855-5863. doi: 10.1021/acsami.6b15444. Epub 2017 Feb 7. PMID: 28117965.
- Wang P , Jiang S , Li Y , Luo Q , Lin J , Hu L , Xu C , Zhu J , Fan L . Fabrication of hypoxia-responsive and uperconversion nanoparticles-modified RBC micro-vehicles for oxygen delivery and chemotherapy enhancement. Biomater Sci. 2020 Aug 21;8(16):4595-4602. doi: 10.1039/d0bm00678e. Epub 2020 Jul 23. PMID: 32700684.
- Anselmo AC, Gupta V, Zern BJ, Pan D, Zakrewsky M, Muzykantov V, Mitragotri S. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013 Dec 23;7(12):11129-37. doi: 10.1021/nn404853z. Epub 2013 Nov 12. PMID: 24182189; PMCID: PMC4128963.
- Brenner JS, Pan DC, Myerson JW, Marcos-Contreras OA, Villa CH, Patel P, Hekierski H, Chatterjee S, Tao JQ, Parhiz H, Bhamidipati K, Uhler TG, Hood ED, Kiseleva RY, Shuvaev VS, Shuvaeva T, Khoshnejad M, Johnston I, Gregory JV, Lahann J, Wang T, Cantu E, Armstead WM, Mitragotri S, Muzykantov V. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat Commun. 2018 Jul 11;9(1):2684. doi: 10.1038/s41467-018-05079-7. PMID: 29992966; PMCID: PMC6041332.
- Zhao Z, Ukidve A, Krishnan V, Fehnel A, Pan DC, Gao Y, Kim J, Evans MA, Mandal A, Guo J, Muzykantov VR, Mitragotri S. Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases. Nat Biomed Eng. 2021 May;5(5):441-454. doi: 10.1038/s41551-020-00644-2. Epub 2020 Nov 16. PMID: 33199847.
- Zhao Z, Kim J, Suja VC, Kapate N, Gao Y, Guo J, Muzykantov VR, Mitragotri S. Red Blood Cell Anchoring Enables Targeted Transduction and Re-Administration of AAV-Mediated Gene Therapy. Adv Sci (Weinh). 2022 Aug;9(24):e2201293. doi: 10.1002/advs.202201293. Epub 2022 Jul 3. PMID: 35780495; PMCID: PMC9404386.
- Ding Y, Lv B, Zheng J, Lu C, Liu J, Lei Y, Yang M, Wang Y, Li Z, Yang Y, Gong W, Han J, Gao C. RBC-hitchhiking chitosan nanoparticles loading methylprednisolone for lung-targeting delivery. J Control Release. 2022 Jan;341:702-715. doi: 10.1016/j.jconrel.2021.12.018. Epub 2021 Dec 18. PMID: 34933051; PMCID: PMC8684098.
- Ferguson LT, Hood ED, Shuvaeva T, Shuvaev VV, Basil MC, Wang Z, Nong J, Ma X, Wu J, Myerson JW, Marcos-Contreras OA, Katzen J, Carl JM, Morrisey EE, Cantu E, Villa CH, Mitragotri S, Muzykantov VR, Brenner JS. Dual Affinity to RBCs and Target Cells (DART) Enhances Both Organ- and Cell Type-Targeting of Intravascular Nanocarriers. ACS Nano. 2022 Mar 22;16(3):4666-4683. doi: 10.1021/acsnano.1c11374. Epub 2022 Mar 10. PMID: 35266686; PMCID: PMC9339245.
- Karami MH, M/Abdouss M, Maleki B. The State of the Art Metal Nanoparticles in Drug Delivery Systems: A Comprehensive Review Nanomed J. 2024. Article in press.
- Hamadani CM, Goetz MJ, Mitragotri S, Tanner EEL. Protein-avoidant ionic liquid (PAIL)-coated nanoparticles to increase bloodstream circulation and drive biodistribution. Sci Adv. 2020 Nov 25;6(48):eabd7563. doi: 10.1126/sciadv.abd7563. PMID: 33239302; PMCID: PMC7688330.
- Blagovic K, Smith C, Ramakrishnan A, Moore L, Soto D, Thompson Z, Stockmann A, Kruszelnicki S, Thakkar A, Murray J, Torres S, Wondimagegnhu B, Yi R, Dadgar M, Paracha A, Page C, Clear L, Chaudhry O, Myint M, Bridgen D, Gilbert J, Seidl K, Sharei A, Loughhead S.
- Bernstein H, Yarar D. Engineered red blood cells (activating antigen carriers) drive potent T cell responses and tumor regression in mice, Front. Immunol. 2022; 13.
- Sun X, Han X, Xu L, Gao M, Xu J, Yang R, Liu Z. Surface-Engineering of Red Blood Cells as Artificial Antigen Presenting Cells Promising for Cancer Immunotherapy. Small. 2017 Oct;13(40). doi: 10.1002/smll.201701864. Epub 2017 Sep 1. PMID: 28861943.
- Sun L, Shen F, Xu J, Han X, Fan C, Liu Z. DNA-Edited Ligand Positioning on Red Blood Cells to Enable Optimized T Cell Activation for Adoptive Immunotherapy. Angew Chem Int Ed Engl. 2020 Aug 24;59(35):14842-14853. doi: 10.1002/anie.202003367. Epub 2020 Jun 9. PMID: 32395895.
- Lorentz KM, Kontos S, Diaceri G, Henry H, Hubbell JA. Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase. Sci Adv. 2015 Jul 17;1(6):e1500112. doi: 10.1126/sciadv.1500112. PMID: 26601215; PMCID: PMC4646778.
- Ukidve A, Zhao Z, Fehnel A, Krishnan V, Pan DC, Gao Y, Mandal A, Muzykantov V, Mitragotri S. Erythrocyte-driven immunization via biomimicry of their natural antigen-presenting function. Proc Natl Acad Sci U S A. 2020 Jul 28;117(30):17727-17736. doi: 10.1073/pnas.2002880117. Epub 2020 Jul 14. PMID: 32665441; PMCID: PMC7395435.
- Cremel M, Guérin N, Horand F, Banz A, Godfrin Y. Red blood cells as innovative antigen carrier to induce specific immune tolerance. Int J Pharm. 2013 Feb 25;443(1-2):39-49. doi: 10.1016/j.ijpharm.2012.12.044. Epub 2013 Jan 7. PMID: 23305866.