More Information
Submitted: November 04, 2023 | Approved: December 12, 2023 | Published: December 13, 2023
How to cite this article: Garavelas A, Michalopoulos E, Mallis P, Nikitos E. Rejuvenation of Ovarian Function after Autologous Platelet Lysate Injection: Promising Evidence from Confirmed Cases. Clin J Obstet Gynecol. 2023; 6: 225-232.
DOI: 10.29328/journal.cjog.1001153
Copyright License: © 2023 Garavelas A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Poor ovarian response; Premature ovarian insufficiency; Platelet iysate; Intraovarian infusion; Follicle stimulating hormone; Pregnancy; Rejuvenation
Rejuvenation of Ovarian Function after Autologous Platelet Lysate Injection: Promising Evidence from Confirmed Cases
Athanasios Garavelas1, Efstathios Michalopoulos2*, Panagiotis Mallis2 and Eros Nikitos1
1Institute of Life, IASO Maternity Hospital, 37-39, Kifissias Avenue, 151 23 Athens, Greece
2Hellenic Cord Blood Bank, Biomedical Research Foundation Academy of Athens, 4 Soranou Efessiou Street,115 27 Athens, Greece
*Address for Correspondence: Efstathios Michalopoulos, Biomedical Research Foundation Academy of Athens, Soranou Efessiou 4, 11527, Athens, Greece
Intraovarian injection of autologous Platelet Lysate (PL) can be considered a potential therapeutic strategy for ovarian function rejuvenation. Especially, in women diagnosed with Poor Ovarian Response (POR) or Primary Ovarian Insufficiency (POI), the exogenous administration of the autologous platelet-derived growth factors, influence positively the regulation of the serum Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH), and Anti-Mullerian Hormone (AMH) and Estradiol (E2). Therefore, the evaluation of the serum levels of the aforementioned hormones was performed on 10 participants with a mean age of 43 ± 4 years diagnosed with POR or POI, who received intraovarian PL injection monotherapy. The monitoring of the serum hormone levels was performed for 3 months. The results of this study clearly showed that all participants were characterized by elevated levels of FSH and LH and reduced levels of E2 and AMH, prior to the PL injection. However, the levels of all hormones started to change after the 1st month of follow-up monitoring. Moreover, 40% of the participants conceived successfully either by natural way or after in vitro Fertilization (IVF). Considering these, the intraovarian injection of autologous PL exhibited promising evidence regarding the altering of hormone levels at physiological values. Moreover, the contained PL growth factors were implicated in angiogenesis promotion and also in toleration of the inflammatory microenvironment, regulating positively ovarian function. In conclusion, the intraovarian autologous PL injection is considered a safe, effective, and tolerable therapeutic strategy in women with POR or POI. Moreover, the results of this study were very encouraging, especially for the women with infertility issues, who want their genetic offspring.
Injection of autologous blood derivatives, such as Platelet-Rich Plasma (PRP) or PL is considered an efficient method for the improvement of ovarian dysfunction, such as POR or POI [1]. Apparently, 1% of the women with an age less than 40 years old, have reported irregular menstrual cycles, and difficulties in fertilization accompanied by a significant reduction in ovarian reservoir [2-5]. Besides the aforementioned issues, at present the donation of oocytes represents the only available strategy for women with POI to acquire their genetic offspring [3]. On the other hand, POR represents a gynecological situation where ovarian stimulation with the use of exogenous administrated hormones is most times low, which further leads to a limited number of produced oocytes [2]. In addition, the majority of the therapeutic protocols for the improvement of ovarian function in women with POR are characterized by a low success rate and therefore by a reduced possibility for conception. Both POI and POR are considered the primary reasons for the increased number of IVF cycles, which are performed in developed countries [6,7]. In the context of assisted reproduction, in Greece, more than 200,000 couples have issues with natural conception, and according to the statistics provided by the Independent Medically Assisted Reproduction Authority, more than 30,000 cycles are performed annually [6,8]. Moreover, an increase of 8% in IVF cycles has been reported since 2014 [6,8]. The above statistics can be further explained by the fact that the modern way of life has caused women to give birth to their first child after 30 years old [9]. Indeed, considering that female infertility is actively associated with several factors, such as daily stress issues, a decrease of oocytes due to aging, or other pathological situations, such as endocrine dysfunction, the existence of endometriosis, polycystic ovary syndrome (PCOS), failure of embryo implantation or other, may explain the reason for the high number of performed IVF cycles [9-11].
In this way, ovarian rejuvenation by establishing alternative therapeutic strategies to increase the production and release of follicles from the available reservoir, should further be evaluated. Hence, it is known that folliculogenesis is driven by specific hormones, such as FSH and LH primarily for follicle production and AMH and LH lately for their maturation and ovulation [12-16]. Besides them, other growth factors, such as transforming growth factor-β1 (TGF-β1), Fibroblast Growth Factor (FGF), Vascular Endothelial Growth Factor (VEGF), Platelet-Derived Growth Factor (PDGF), Hepatocyte Growth Factor (HGF) and others, have been implicated in several steps of folliculogenesis, such as the transition from primordial to pre-antral follicles, or further survival and maturation of antral follicles [17-19]. For this purpose, the rejuvenation of ovarian function by using the autologous PRP could increase oocyte production and thus may contribute to a higher chance of pregnancy succession. In literature, there is a great number of studies where the potential of the intraovarian injection of autologous PRP in inducing rejuvenation of ovarian function, has been shown [20-27]. PRP is a rich source of growth factors that are released by the activated platelets [28,29]. On the other hand, PL is considered also an equal and safe source of autologous growth factors, offering the advantage of storage and delivery at specific time points, limiting in this way the long waiting time (required for PRP preparation) of the patient at the clinic [30].
In this way, the primary aim of this study was the investigation of the beneficial impact of intraovarian PL injection in women with POR or POI. To achieve this, specific biochemical parameters including FSH, LH, AMH, and E2 were evaluated prior to and post-PL injections. Therefore, the acquired data may shed light on the potential relationship between the ovarian rejuvenation mechanism and the exogenous provided growth factors, which can result in improving ovarian function in women with POI or POR.
Study design
This whole study was performed between September 2022 and June 2023 at the Institute of Life, IASO Hospital, Athens, Greece. Initially, 10 participants ages 35 years - 49 years, with diagnosed POR or POI were enrolled, based on ESHRE guidelines and Bologna criteria. The study has been approved by the Scientific Board of “IASO” Maternity Hospital with registration number 021023. The study followed the regulation as outlined by the declaration of Helsinki and is in accordance with the Greek Bioethics Committee of Human Reproduction. All participants were informed of the purpose of the study and signed the consent before the initiation of the study. As exclusion criteria, the presence of autoimmune disorders, chronic inflammatory diseases, sexually transmitted infectious diseases, tubal factor infertility, tubal obstruction, thyroid dysfunction, endometriosis, hematological disorders, cardiovascular disease, and body mass index (BMI) > 30 or < 18.5, gynecological cancer, were considered prior the selection of the participants.
Participants examination
Routine examination of the reproductive dynamic of the participants involved the biochemical evaluation of serum levels of FSH, LH, AMH, and E2. The evaluation of the above levels was performed on day 3 of the menstrual cycle. The quantification of the hormones was performed using the chemiluminescent microparticle immunoassay (Roche Diagnostics GmbH, Mannheim, Germany) using the Roche analyzer (Roche Cobas 4111, Basel, Switzerland).
Platelet Lysate production protocol
A quantity of 20 ml was collected from all participants enrolled in this study. The production protocol of PRP was performed using a two-step centrifugation process at 160 g and 480 g for 20 min each, respectively. All centrifugation steps were performed at room temperature. After the first centrifugation step, an amount of 6 ml of plasma was transferred to a new falcon (BD Biosciences, Franklin Lakes, NJ, USA), followed by the second centrifugation. Finally, the supernatant was removed, and the remaining 4 mL of PRP were isolated. To obtain the PL, PRP was left in the freezer at -80 oC for a minimum of 48 hours. After this period, the PL was rapidly thawed at 37 oC, filtered with 0.45 μm filter, and was ready for intraovarian infusion. To confirm the production of PRP and PL, Platelet (PLT) count was performed before and after the performance of the production protocol.
Growth factors quantification
The growth factor content of peripheral blood, PRP, and PL were quantified using commercial ELISA kits. The determination of TGF-β1, PDGF-AA, PDGF-BB, FGF, VEGF-A, and HGF with the ELISA method was performed following the manufacturer’s instructions (OriGene Technologies, Rockville, MD, USA).
Intraovarian infusion of PL
All participants discontinued the Hormone Replacement Therapy (HRT) for at least 6 months before the initiation of the study. Transvaginal ultrasound monitoring was used to guide properly the intraovarian infusion of PL. Briefly, in each ovary, an intramedullary injection of 1 ml PL was performed using a 17-gauge single-lumen needle, on multiple sites. After the injections, participants remained in the supine position for 15 min, before leaving the maternity clinic.
Follow up monitoring
The assessment of the PL intraovarian infusion in rejuvenating the ovarian function included a two-month follow-up monitoring. Biochemical examination of the levels of FSH, LH, AMH, and E2 was performed for two constitutive menstrual cycles. All hormone levels were determined on day 3 of the menstrual cycle. A positive outcome was considered in the classification of participants as non-POR, POI, or pre-menopause. Also, the regularity of the menstrual cycle was considered during the study enrollment.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism v.6.0.1 (GraphPad Software, San Diego, CA, USA). Data were analyzed using non-parametric tests, such as the Kruskal Wallis and Matt-Whitney test. Statistically significant differences between values were considered when the p - value was less than 0.05. All values were presented as mean ± standard deviation.
Evaluation of PL production protocol
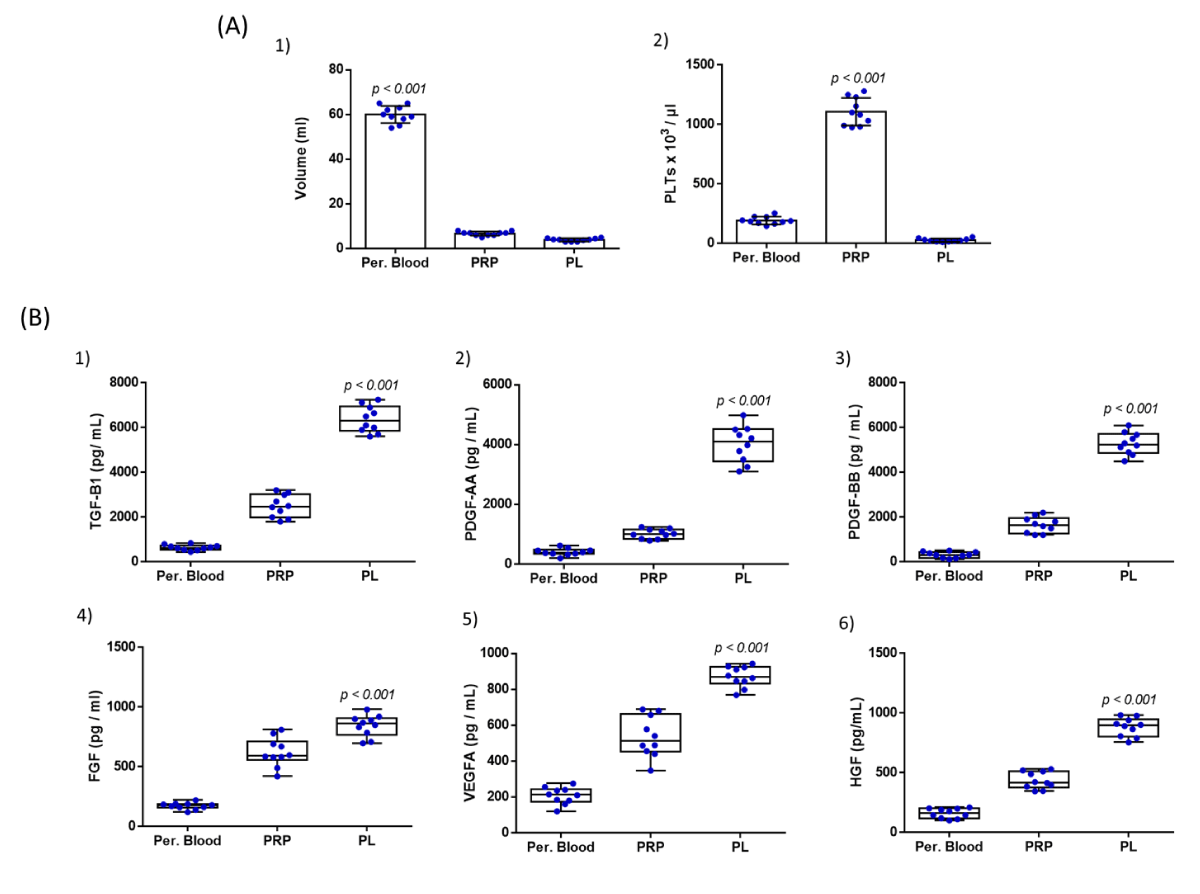
The peripheral blood sample (20 ml) was efficiently taken from all participants, which led to the successful production of PRP and eventually PL. Specifically, the volume of the obtained PRP was 6.7 ± 0.9 mL, which resulted in the production of 3.9 ± 0.7 mL of filtered PL. Moreover, the average PLT count of all participants was 192 ± 31 x 103 / μL, 1107 ± 110 x 103 / μL, and 28 ± 13 x 103 / μL, for the peripheral blood, PRP and PL, respectively (Figure 1). The PLT recovery in PRP was 64%. Statistically significant differences were observed between the volume and PLT concentration between peripheral blood, PRP, and PL samples (p < 0.001).
Figure 1: Quality characteristics of PL involved the determination of volume and PLT count (A) and the biomolecule content quantification (B), in peripheral blood, PRP, and PL samples. Volume determination (A1) and PLT count in peripheral blood (A2), PRP, and PL samples from all participants. Statistically, significant differences were observed between the volume and PLT concentrations between peripheral blood, PRP, and PL samples (p < 0.001). The quantification involved the determination of TGF-β1 (B1), PDGF-AA (B2), PDGF-BB (B3), FGF (B4), VEGF-A (B5), and HGF (B6). Kruskall-Wallis test indicated statistically significant differences between the peripheral blood, PRP, and PL with respect to TGF-B1 (p < 0.001), PDGF-AA (p < 0.001), PDGF-BB (p < 0.001), FGF (p < 0.001), VEGF-A (p < 0.001) and HGF (p < 0.001).
The growth factor content of PL was determined using the ELISA method. Specifically, the levels of TGF-b1 were 644 ± 115 pg/ mL, 2493 ± 478 pg/mL, and 6372 ± 564 pg/mL for the peripheral blood, PRP and PL samples (Figure 1). The levels of PDGF-AA were 410 ± 113 pg/mL, 1014 ± 150 pg/mL, and 4014 ± 574 pg/mL, the levels of PDGF-BB were 319 ± 127 pg/mL, 1650 pg/mL and 5288 ± 467 pg/mL, the levels of FGF were 171 ± 26 pg/mL, 620 ± 114 pg/mL and 843 ± 85 pg/mL, for the peripheral blood, PRP and PL samples, respectively (Figure 1). The levels of VEGF-A were 207 ± 44, 536 ± 107, and 870 ± 54 and the levels of HGF were 160 ± 38, 436 ± 67, and 882 ± 73 pg/mL for the same samples (Figure 1). Statistical analysis revealed significant differences for all growth factors between peripheral blood, PRP, and PL samples (p < 0.001).
Evaluation of the improvement of ovarian function
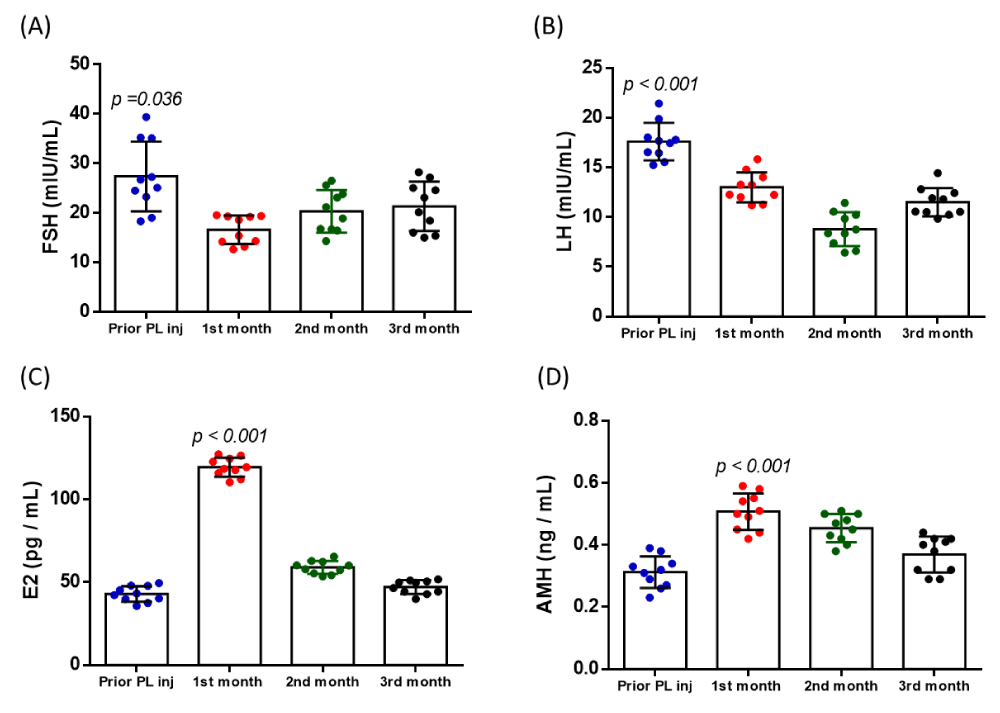
A total of 10 women with the diagnosis either of POI or POR, were recruited for this study. The mean age of the participants was 43 ± 4 years and the BMI was 25 ± 3 kg/m2. In addition, all participants failed to conceive either after IVF, ICSI, or IUI, in the past (Table 1). Furthermore, to evaluate the impact of PL on ovarian function rejuvenation, determination of serum hormone levels of FSH, LH, E2, and AMH were determined prior to the PL injection and within the 1st, 2nd, and 3rd month post-PL injection. Specifically, the levels of FSH were 27.4 ± 6.7 mIU/mL, 16.5 ± 2.7 mIU/mL, 20.3 ± 4.1 mIU/mL, and 21.3 ± 4.7 mIU/mL, prior to PL injection, and after the 1st, 2nd and 3rd month, respectively (Figure 2, Table S1 and S2). The levels of LH were 17.6 ± 1.8 mIU/mL, 13.1 ± 1.4 mIU/mL, 8.7 ± 1.6 mIU/mL, and 11.5 ± 1.3 mIU/mL, for the same period of time (Figure 2, Table S1 and S2). The levels of E2 were 42.8 ± 4.4 pg/mL, 119.4 ± 5.5 pg/mL, 58.8 ± 3.7 pg/mL and 47.1 ± 3.8 pg/mL and the levels of AMH were 0.31 ± 0.05 ng/mL, 0.51 ± 0.06 ng/mL, 0.45 ± 0.04 ng/mL and 0.37 ± 0.06 ng/mL, prior and post PL injection (1st, 2nd and 3rd month), respectively (Figure 2, Table S1 and S2). Elevation of all hormone levels was observed within the 1st month, after the intraovarian PL injection in all participants. A gradual decrease of all hormone levels was observed in all participants, after the 2nd and 3rd month (Table S1 and S2). Statistically significant differences were observed in FSH (p = 0.036), LH (p < 0.001), E2 (p < 0.001), and AMH (p < 0.001) before and after the intraovarian PL injection. Importantly, besides the elevation of the serum hormone levels, 4 out of 10 participants successfully conceived naturally or after the IVF cycle, which resulted in successful live births (Table 1).
Figure 2: Determination of FSH, LH, E2, and AMH serum hormone levels. The levels of FSH (A), LH (B), E2 (C), and AMH (D) were determined before the PL injection and after the 1st, 2nd, and 3rd-month post-injection. Statistically significant differences were observed between FSH (p = 0.036), LH (p < 0.001), E2 (p < 0.001), and AMH (p < 0.001) prior to and post the PL injection.
| Table 1: Clinical features of all participants enrolled in this study. 40% of the participants got pregnant either with natural conception or after the IVF cycle. | |||||
| Participant No | Age (years) |
BMI (kg/m2) | Successful Pregnancy (prior PL injection) | No previous ART failure | Successful Pregnancy (post PL injection) |
| 1 | 43 | 23.12 | Negative | IVF: 1 | Negative |
| 2 | 48 | 32.01 | Negative | IVF:3 | Negative |
| 3 | 36 | 28.74 | Negative | ICSI:2 | Positive (with natural conceive) |
| 4 | 39 | 25.32 | Negative | IVF:2 IUI:1 |
Negative |
| 5 | 48 | 24.78 | Negative | IVF:6 IUI:4 |
Positive (after IVF cycle) |
| 6 | 44 | 26.75 | Negative | IVF:5 ICSI:2 |
Positive (after IVF cycle) |
| 7 | 44 | 25.34 | Negative | IVF:1 IUI:2 |
Positive (after IVF cycle) |
| 8 | 41 | 21.83 | Negative | IVF:3 | Negative |
| 9 | 40 | 22.76 | Negative | IVF:2 ICSI:3 |
Negative |
| 10 | 44 | 24.12 | Negative | IVF:4 IUI:1 |
Negative |
| ICSI: Intracytoplasmic Sperm Injection, IUI: Intrauterine Insemination. | |||||
| Table S1: Determination of serum hormone levels before and after the PL injection. | |||||
| Hormones | Prior PL injection | 1st month post | 2nd month post | 3rd month post | p - value |
| FSH (mIU/mL) | 27.4 ± 6.7 | 16.5 ± 2.7 | 20.3 ± 4.1 | 21.3 ± 4.7 | < 0.036 |
| LH (mIU/mL) | 17.6 ± 1.8 | 13.1 ± 1.4 | 8.7 ± 1.6 | 11.5 ± 1.3 | < 0.001 |
| E2 (pg/mL) | 42.8 ± 4.4 | 119.4 ± 5.5 | 58.8 ± 3.7 | 47.1 ± 3.8 | < 0.001 |
| AMH (ng/mL) | 0.31 ± 0.05 | 0.51 ± 0.06 | 0.45 ± 0.04 | 0.37 ± 0.06 | < 0.001 |
| Table S2. Clinical features of all participants enrolled in this study. Serum hormone levels of FSH, LH, E2 and AMH were determined prior the PL injection and after the 1st, 2nd and 3rd month after the PL injection. | ||||||||||||||||
| FSH (mIU/mL) | LH (mIU/mL) | E2 (pg/mL) | AMH (ng/mL) | |||||||||||||
| Part.No | Prior PL injection | 1st month post | 2nd month post | 3rd month post | Prior PL injection | 1st month post | 2nd month post | 3rd month post | Prior PL injection | 1st month post | 2nd month post | 3rd month post | Prior PL injection | 1st month post | 2nd month post | 3rd month post |
| 1 | 19.1 | 15.4 | 16.7 | 16.0 | 18 | 12.25 | 7.38 | 10.23 | 45.2 | 127.2 | 65.4 | 51.1 | 0.34 | 0.51 | 0.45 | 0.32 |
| 2 | 18.3 | 14.2 | 16.4 | 15.0 | 17.45 | 11.2 | 8.35 | 10.5 | 40.2 | 110.3 | 60.2 | 50.4 | 0.23 | 0.42 | 0.4 | 0.29 |
| 3 | 35.2 | 13.2 | 14.3 | 15.4 | 17.68 | 13.23 | 6.59 | 12.8 | 47.8 | 115.8 | 55.3 | 49.8 | 0.27 | 0.45 | 0.43 | 0.29 |
| 4 | 25.1 | 19.3 | 21.2 | 23.1 | 16.45 | 11.25 | 8.75 | 11.9 | 39.8 | 119.4 | 57.8 | 44.3 | 0.31 | 0.49 | 0.42 | 0.38 |
| 5 | 27.2 | 19.5 | 23.8 | 25.1 | 17.79 | 15.82 | 8.37 | 11.8 | 37.4 | 122.6 | 53.4 | 42.8 | 0.38 | 0.54 | 0.5 | 0.44 |
| 6 | 24.5 | 12.6 | 18.9 | 20.1 | 21.42 | 12 | 6.42 | 9.85 | 42.1 | 124.5 | 54.2 | 46.4 | 0.39 | 0.55 | 0.51 | 0.42 |
| 7 | 23.2 | 14.3 | 16.7 | 18.4 | 19.87 | 14 | 10.56 | 12.43 | 43.2 | 126.4 | 62.8 | 44.3 | 0.29 | 0.52 | 0.47 | 0.41 |
| 8 | 26.7 | 19.2 | 23.1 | 24.6 | 16.54 | 14.78 | 11.42 | 10.56 | 35.6 | 117.6 | 60.1 | 39.8 | 0.26 | 0.44 | 0.38 | 0.32 |
| 9 | 35.1 | 19.4 | 26.5 | 28.2 | 15.54 | 13.27 | 10.21 | 14.42 | 47.9 | 118.4 | 62.3 | 49.6 | 0.33 | 0.59 | 0.53 | 0.42 |
| 10 | 39.4 | 18.6 | 25.6 | 27.2 | 15.23 | 12.23 | 9.85 | 10.53 | 49.4 | 112.0 | 57.3 | 51.7 | 0.32 | 0.58 | 0.48 | 0.45 |
In the present study, we encouraged to evaluate the possibility of ovarian function rejuvenation after intraovarian autologous PL injection. The whole study involved 10 cases with diagnosed POI or POR, who have failed previously for successful conception either under natural or assisted reproduction way. For this purpose, peripheral blood samples were acquired from each participant, to produce the PL enriched in growth factors, to be used for intraovarian infusion. Before, the administration of PL, an analysis of the quality characteristics was performed to clearly indicate the absence of PLTs and the presence of platelet-derived growth factors. Indeed, using the ELISA method, it was determined that autologous PL was a rich source of TGF-β1, PDGF-AA/ BB, FGF, VEGF-A, and HGF. In the past, peripheral blood derivatives, such as PRP and PL, have been shown to exert key regeneration properties in several applications [31-38]. Moreover, PL offers the advantage of storage at low temperatures for more than a month, without altering the growth factor content. In this way, the mean waiting time of each participant at the clinic was significantly reduced, inducing less anxiety and stressful events, which may further negatively impact the disease pathogenesis.
The intraovarian PL infusion was performed with ultrasound assistance, and no side effects or severe pain was reported by the participants either during or after the PL injection or within the 3 months follow-up. Considering the obtained data, in all cases it was observed a decrease in FSH and LH with a parallel increase of E2 and AMH within the 1st month of follow-up. However, the elevated hormone levels started to decrease after the 2nd month. In 3rd month the hormone levels tended to reach the initial ones (prior to the PL injection), suggesting possibly a 2nd intraovarian PL injection may be required, to retain the hormones at higher levels. However, during this study, participants were encouraged to perform sexual intercourse, which resulted in one pregnancy. Within the 3-month follow-up period, 3 more pregnancies were detected after IVF monocycle. In this way, following the PL injections, 40% of the participants conceived successfully and also gave birth to physiological infants. The beneficial impact of PL injection in ovarian function restoration has been also confirmed by previously performed studies by our group [20] and also by Pantos et. al [39,40]. The fact that all participants experienced elevated hormone levels during the performance of this study, clearly showed that PL had a beneficial effect in ovarian function restoration mechanism.
Indeed, the PL growth factors, such as TGF-β1, PDGF-AA/ BB, FGF, VEGF-A, and HGF play major roles in tissue regeneration and wound healing. It is known that the molecular network responsible for angiogenesis has been significantly disrupted in women with POI or POR [41-43]. In this way, the exogenous administration of PDGF-AA/BB, VEGF-A, and TGF-β1 may result in the activation of the angiogenesis and neo-angiogenesis, thus the ovarian function may be improved and hence can better support the production of large ovulatory antral follicles [41,44].
Furthermore, in our study after the intraovarian PL administration in women with POR or POI and following a three-month examination, it was observed that the hormone levels in most of the women restored to normal levels. This may suggest that the PL growth factors, besides the impact they may have stimulating specific intracellular signaling pathways (e.g. proliferation, migration, protein secretion), may also positively affect the neuroendocrine system of the Hypothalamus Pituitary Adrenal (HPA) axis [45]. This phenomenon is further confirmed by other researchers in the field. Indeed, Mikhael, et al. [45] have reported that this set of growth factors besides the direct impact on ovarian function, can also positively influence the HPA axis, to induce better control of the FSH, LH, E2, and AMH levels. However, to date, the exact mechanism by which PL can affect the HPA axis system has not been comprehensively clarified [8,20]. More experimental procedures involving the use of animal models, in order to obtain representative tissue biopsies, are required to be performed, to acquire safe conclusions.
In addition, the presence of a high concentration of the HGF was also confirmed in the injected autologous PL samples, suggesting possibly the existence of a broader immunomodulatory mediated mechanism for ovarian function restoration [34]. Indeed, HGF is a potent immunomodulatory molecule, which can tolerate the overactivated immune responses exerted by the stimulated M1 macrophages, Th1 cells, Dendritic Cells (DCs), and B cells. In this way, HGF alongside other immunoregulatory biomolecules, such as galectins, and indoleamine 2,3 dioxygenase may favor the anti-inflammatory responses in infertile women, which their condition may further be related to the presence of autoimmune disorders (e.g. Thyroid autoimmunity) or other idiopathic reasons related to the adaptation of an inflammatory microenvironment. However, the production of functional primordial follicles represents a complex procedure, where other factors including daily stress, way of life (healthy nutrition, exercise, absence of smoking), working hours as well as the genetic background (e.g presence of mutations or epigenetic alterations) and underlying disorders may influence either positively or negatively the mitotic activity of the primordial follicles [10-14]. For this reason, in the near future, more studies should be performed, which will include both the individual’s genetic background accompanied by the evaluation of the hormone levels, to better decipher the POR or POI pathogenetic mechanism. By acquiring more data for the abovementioned disorders, the clinicians may precisely develop better therapeutic strategies, considering also the PL benefits, for women with POR or POI who want to have their genetic offspring. This further brings personalized medicine one step closer to its clinical utility, thus significantly improving the way of life of couples with difficulties in successful conception.
Considering the data presented herein, the intraovarian PL injection has a positive impact on ovarian function restoration in women with diagnosed POR or POI. However, the proper preparation and administration of peripheral blood products, such as PRP and PL is strongly suggested to be performed in licensed laboratories with well-trained physicians. Moreover, the PL production process is considered under the term of 21 CFR “minimally manipulation”, which involves only the centrifugation and freeze/thawing step for its preparation [20]. On the contrary, PRP requires the initial activation of the contained PLTs to release the growth factors, utilizing exogenous factors, such as calcium gluconate, batroxobin, and others. In return, the use of exogenous factors may result in an altered PLT activating profile, resulting in variations in growth factor levels. On the other hand, PL is considered a safer and equally effective solution compared to PRP, where no exogenous activating factors are required, thus further fulfilling the requirement of “minimally manipulated” therapy, outlined by 21 CFR. Moreover, in order to acquire the best outcome for the participants, a few important parameters, such as the PLTs concentration and the growth factor levels in the produced PL, should be verified before the administration to the patients.
In conclusion, the intraovarian autologous PL infusion is considered a safe, and tolerable approach to promote the rejuvenation of ovarian function. However, more research studies are required, to decipher completely the underlying mechanism of ovarian function rejuvenation, which represents the “holy grail” of reproductive medicine. Undoubtedly, PL should be considered an effective “off-the-self” therapeutic approach with encouraging results in women with infertility issues.
Data availability statement
Data and materials that support the results presented in this paper are freely available upon request.
- Panda SR, Sachan S, Hota S. A Systematic Review Evaluating the Efficacy of Intra-Ovarian Infusion of Autologous Platelet-Rich Plasma in Patients With Poor Ovarian Reserve or Ovarian Insufficiency. Cureus. 2020 Dec 12;12(12):e12037. doi: 10.7759/cureus.12037. PMID: 33457137; PMCID: PMC7797441.
- Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005 Jul-Aug;11(4):391-410. doi: 10.1093/humupd/dmi012. Epub 2005 May 26. PMID: 15919682.
- De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010 Sep 11;376(9744):911-21. doi: 10.1016/S0140-6736(10)60355-8. Epub 2010 Aug 11. PMID: 20708256.
- Cohen J, Chabbert-Buffet N, Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder--a plea for universal definitions. J Assist Reprod Genet. 2015 Dec;32(12):1709-12. doi: 10.1007/s10815-015-0595-y. Epub 2015 Oct 13. PMID: 26463876; PMCID: PMC4681731.
- Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011 Jul;26(7):1616-24. doi: 10.1093/humrep/der092. Epub 2011 Apr 19. PMID: 21505041.
- Fragoulakis V, Maniadakis N. Estimating the long-term effects of in vitro fertilization in Greece: an analysis based on a lifetime-investment model. Clinicoecon Outcomes Res. 2013 Jun 20;5:247-55. doi: 10.2147/CEOR.S44784. PMID: 23818800; PMCID: PMC3694799.
- Chae-Kim JJ, Gavrilova-Jordan L. Premature Ovarian Insufficiency: Procreative Management and Preventive Strategies. Biomedicines. 2018 Dec 28;7(1):2. doi: 10.3390/biomedicines7010002. PMID: 30597834; PMCID: PMC6466184.
- Sfakianoudis K, Simopoulou M, Rapani A, Grigoriadis S, Maziotis E, Giannelou P, Pantou A, Vaxevanoglou T, Fakiridou M, Koutsilieris M, Pantos K. The Impact of the Economic Recession in Greece on Assisted Reproduction Demand: A Retrospective Longitudinal Study. Medicina (Kaunas). 2019 Sep 29;55(10):654. doi: 10.3390/medicina55100654. PMID: 31569483; PMCID: PMC6843187.
- Women are having their first child at an older age - Products Eurostat News – Eurostat. https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20200515-2.
- Shelling AN, Ahmed Nasef N. The Role of Lifestyle and Dietary Factors in the Development of Premature Ovarian Insufficiency. Antioxidants (Basel). 2023 Aug 11;12(8):1601. doi: 10.3390/antiox12081601. PMID: 37627595; PMCID: PMC10451748.
- Ebrahimi M, Akbari Asbagh F. Pathogenesis and causes of premature ovarian failure: an update. Int J Fertil Steril. 2011 Jul;5(2):54-65. Epub 2011 Sep 23. PMID: 24963360; PMCID: PMC4059950.
- Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58(1):44-50. doi: 10.1262/jrd.2011-012. PMID: 22450284.
- Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian Folliculogenesis. Results Probl Cell Differ. 2016;58:167-90. doi: 10.1007/978-3-319-31973-5_7. PMID: 27300179.
- Yang DZ, Yang W, Li Y, He Z. Progress in understanding human ovarian folliculogenesis and its implications in assisted reproduction. J Assist Reprod Genet. 2013 Feb;30(2):213-9. doi: 10.1007/s10815-013-9944-x. PMID: 23388838; PMCID: PMC3585681.
- Vegetti W, Alagna F. FSH and folliculogenesis: from physiology to ovarian stimulation. Reprod Biomed Online. 2006 Jun;12(6):684-94. doi: 10.1016/s1472-6483(10)61080-2. PMID: 16792843.
- Fadini R, Coticchio G, Brambillasca F, Mignini Renzini M, Novara PV, Brigante C, De Ponti E, Dal Canto M. Clinical outcomes from mature oocytes derived from preovulatory and antral follicles: reflections on follicle physiology and oocyte competence. J Assist Reprod Genet. 2015 Feb;32(2):255-61. doi: 10.1007/s10815-014-0386-x. Epub 2014 Dec 2. PMID: 25449291; PMCID: PMC4354189.
- Erickson GF, Shimasaki S. The physiology of folliculogenesis: the role of novel growth factors. Fertil Steril. 2001 Nov;76(5):943-9. doi: 10.1016/s0015-0282(01)02859-x. PMID: 11704115.
- Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009 Jan;27(1):14-23. doi: 10.1055/s-0028-1108006. Epub 2009 Feb 5. PMID: 19197801; PMCID: PMC2947191.
- Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update. 2005 Mar-Apr;11(2):162-77. doi: 10.1093/humupd/dmi001. Epub 2005 Feb 10. PMID: 15705959.
- Garavelas A, Mallis P, Michalopoulos E, Nikitos E. Clinical Benefit of Autologous Platelet-Rich Plasma Infusion in Ovarian Function Rejuvenation: Evidence from a Before-After Prospective Pilot Study. Medicines (Basel). 2023 Feb 27;10(3):19. doi: 10.3390/medicines10030019. PMID: 36976308; PMCID: PMC10056078.
- Rickers NS, Sills ES. Is autologous platelet activation the key step in ovarian therapy for fertility recovery and menopause reversal? Biomedicine (Taipei). 2022 Dec 1;12(4):1-8. doi: 10.37796/2211-8039.1380. PMID: 36816178; PMCID: PMC9910228.
- Sharara FI, Lelea LL, Rahman S, Klebanoff JS, Moawad GN. A narrative review of platelet-rich plasma (PRP) in reproductive medicine. J Assist Reprod Genet. 2021 May;38(5):1003-1012. doi: 10.1007/s10815-021-02146-9. Epub 2021 Mar 15. PMID: 33723748; PMCID: PMC8190208.
- Ahmadian S, Sheshpari S, Pazhang M, Bedate AM, Beheshti R, Abbasi MM, Nouri M, Rahbarghazi R, Mahdipour M. Intra-ovarian injection of platelet-rich plasma into ovarian tissue promoted rejuvenation in the rat model of premature ovarian insufficiency and restored ovulation rate via angiogenesis modulation. Reprod Biol Endocrinol. 2020 Aug 5;18(1):78. doi: 10.1186/s12958-020-00638-4. PMID: 32758249; PMCID: PMC7405361.
- Cakiroglu Y, Saltik A, Yuceturk A, Karaosmanoglu O, Kopuk SY, Scott RT, Tiras B, Seli E. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging (Albany NY). 2020 Jun 5;12(11):10211-10222. doi: 10.18632/aging.103403. Epub 2020 Jun 5. PMID: 32507764; PMCID: PMC7346073.
- Park HS, Ulin M, Cetin E. Ovarian Rejuvenation Using Platelet-Rich Plasma: a Promising Option for Women in Early Menopause to Have a Baby. Reprod Sci. 2020 Nov;27(11):1983-1984. doi: 10.1007/s43032-020-00315-2. Epub 2020 Sep 15. PMID: 32935255.
- Aflatoonian A, Lotfi M, Saeed L, Tabibnejad N. Effects of Intraovarian Injection of Autologous Platelet-Rich Plasma on Ovarian Rejuvenation in Poor Responders and Women with Primary Ovarian Insufficiency. Reprod Sci. 2021 Jul;28(7):2050-2059. doi: 10.1007/s43032-021-00483-9. Epub 2021 Mar 8. PMID: 33683669.
- Petryk N, Petryk M. Ovarian Rejuvenation Through Platelet-Rich Autologous Plasma (PRP)-a Chance to Have a Baby Without Donor Eggs, Improving the Life Quality of Women Suffering from Early Menopause Without Synthetic Hormonal Treatment. Reprod Sci. 2020 Nov;27(11):1975-1982. doi: 10.1007/s43032-020-00266-8. Epub 2020 Jul 22. PMID: 32700285.
- Mallis P, Michalopoulos E, Balampanis K, Sarri EF, Papadopoulou E, Theodoropoulou V, Georgiou E, Kountouri A, Lambadiari V, Stavropoulos-Giokas C. Investigating the production of platelet lysate obtained from low volume Cord Blood Units: Focus on growth factor content and regenerative potential. Transfus Apher Sci. 2022 Dec;61(6):103465. doi: 10.1016/j.transci.2022.103465. Epub 2022 May 20. PMID: 35623959.
- Pavlovic V, Ciric M, Jovanovic V, Stojanovic P. Platelet Rich Plasma: a short overview of certain bioactive components. Open Med (Wars). 2016 Aug 12;11(1):242-247. doi: 10.1515/med-2016-0048. PMID: 28352802; PMCID: PMC5329835.
- Klatte-Schulz F, Schmidt T, Uckert M, Scheffler S, Kalus U, Rojewski M, Schrezenmeier H, Pruss A, Wildemann B. Comparative Analysis of Different Platelet Lysates and Platelet Rich Preparations to Stimulate Tendon Cell Biology: An In Vitro Study. Int J Mol Sci. 2018 Jan 10;19(1):212. doi: 10.3390/ijms19010212. PMID: 29320421; PMCID: PMC5796161.
- Protogerou V, Beshari SE, Michalopoulos E, Mallis P, Chrysikos D, Samolis AA, Stavropoulos-Giokas C, Troupis T. The Combined Use of Stem Cells and Platelet Lysate Plasma for the Treatment of Erectile Dysfunction: A Pilot Study-6 Months Results. Medicines (Basel). 2020 Mar 18;7(3):14. doi: 10.3390/medicines7030014. PMID: 32197323; PMCID: PMC7151592.
- Protogerou V, Michalopoulos E, Mallis P, Gontika I, Dimou Z, Liakouras C, Stavropoulos-Giokas C, Kostakopoulos N, Chrisofos M, Deliveliotis C. Administration of Adipose Derived Mesenchymal Stem Cells and Platelet Lysate in Erectile Dysfunction: A Single Center Pilot Study. Bioengineering (Basel). 2019 Mar 5;6(1):21. doi: 10.3390/bioengineering6010021. PMID: 30841525; PMCID: PMC6466012.
- Rebulla P, Pupella S, Santodirocco M, Greppi N, Villanova I, Buzzi M, De Fazio N, Grazzini G; Italian Cord Blood Platelet Gel Study Group (see Appendix 1). Multicentre standardisation of a clinical grade procedure for the preparation of allogeneic platelet concentrates from umbilical cord blood. Blood Transfus. 2016 Jan;14(1):73-9. doi: 10.2450/2015.0122-15. Epub 2015 Jul 29. PMID: 26509822; PMCID: PMC4731342.
- Mallis P, Michalopoulos E, Sarri EF, Papadopoulou E, Theodoropoulou V, Katsimpoulas M, Stavropoulos-Giokas C. Evaluation of the Regenerative Potential of Platelet-Lysate and Platelet-Poor Plasma Derived from the Cord Blood Units in Corneal Wound Healing Applications: An In Vitro Comparative Study on Corneal Epithelial Cells. Curr Issues Mol Biol. 2022 Sep 22;44(10):4415-4438. doi: 10.3390/cimb44100303. PMID: 36286018; PMCID: PMC9600746.
- Azmi AF, Yahya MAAM, Azhar NA, Ibrahim N, Ghafar NA, Ghani NAA, Nizar MAM, Yunus SSM, Singh TKL, Law JX, Ng SL. In Vitro Cell Proliferation and Migration Properties of Oral Mucosal Fibroblasts: A Comparative Study on the Effects of Cord Blood- and Peripheral Blood-Platelet Lysate. Int J Mol Sci. 2023 Mar 17;24(6):5775. doi: 10.3390/ijms24065775. PMID: 36982842; PMCID: PMC10058190.
- Ng SL, Azhar NA, Budin SB, Ibrahim N, Abdul Ghani NA, Abd Ghafar N, Law JX. Effects of Platelet Lysate Gels Derived from Different Blood Sources on Oral Mucosal Wound Healing: An In Vitro Study. Gels. 2023 Apr 17;9(4):343. doi: 10.3390/gels9040343. PMID: 37102955; PMCID: PMC10137921.
- Suresh A, Balouch B, Martha VV, Sataloff RT. Laryngeal Applications of Platelet Rich Plasma and Platelet Poor Plasma: A Systematic Review. J Voice. 2021 Aug 9:S0892-1997(21)00232-0. doi: 10.1016/j.jvoice.2021.07.007. Epub ahead of print. PMID: 34384663.
- Mallis P, Michalopoulos E, Balampanis K, Sarri EF, Papadopoulou E, Theodoropoulou V, Georgiou E, Kountouri A, Lambadiari V, Stavropoulos-Giokas C. Investigating the production of platelet lysate obtained from low volume Cord Blood Units: Focus on growth factor content and regenerative potential. Transfus Apher Sci. 2022 Dec;61(6):103465. doi: 10.1016/j.transci.2022.103465. Epub 2022 May 20. PMID: 35623959.
- Sfakianoudis K, Simopoulou M, Nitsos N, Rapani A, Pappas A, Pantou A, Chronopoulou M, Deligeoroglou E, Koutsilieris M, Pantos K. Autologous Platelet-Rich Plasma Treatment Enables Pregnancy for a Woman in Premature Menopause. J Clin Med. 2018 Dec 20;8(1):1. doi: 10.3390/jcm8010001. PMID: 30577435; PMCID: PMC6352170.
- Sfakianoudis K, Simopoulou M, Grigoriadis S, Pantou A, Tsioulou P, Maziotis E, Rapani A, Giannelou P, Nitsos N, Kokkali G, Koutsilieris M, Pantos K. Reactivating Ovarian Function through Autologous Platelet-Rich Plasma Intraovarian Infusion: Pilot Data on Premature Ovarian Insufficiency, Perimenopausal, Menopausal, and Poor Responder Women. J Clin Med. 2020 Jun 10;9(6):1809. doi: 10.3390/jcm9061809. PMID: 32532000; PMCID: PMC7355907.
- Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006 Apr 12;4:18. doi: 10.1186/1477-7827-4-18. PMID: 16611363; PMCID: PMC1459163.
- Ishizuka B. Current Understanding of the Etiology, Symptomatology, and Treatment Options in Premature Ovarian Insufficiency (POI). Front Endocrinol (Lausanne). 2021 Feb 25;12:626924. doi: 10.3389/fendo.2021.626924. PMID: 33716979; PMCID: PMC7949002.
- Ferrari AR, Cortrezzi S, Borges E Junior, Braga D, Souza MDCB, Antunes RA. Evaluation of the Effects of Platelet-Rich Plasma on Follicular and Endometrial Growth: A Literature Review. JBRA Assist Reprod. 2021 Oct 4;25(4):601-607. doi: 10.5935/1518-0557.20210036. PMID: 34415119; PMCID: PMC8489815.
- McFee RM, Rozell TG, Cupp AS. The balance of proangiogenic and antiangiogenic VEGFA isoforms regulate follicle development. Cell Tissue Res. 2012 Sep;349(3):635-47. doi: 10.1007/s00441-012-1330-y. Epub 2012 Feb 10. PMID: 22322423; PMCID: PMC3429770.
- Mikhael S, Punjala-Patel A, Gavrilova-Jordan L. Hypothalamic-Pituitary-Ovarian Axis Disorders Impacting Female Fertility. Biomedicines. 2019 Jan 4;7(1):5. doi: 10.3390/biomedicines7010005. PMID: 30621143; PMCID: PMC6466056.