More Information
Submitted: November 15, 2023 | Approved: November 27, 2023 | Published: November 28, 2023
How to cite this article: Karami MH, Abdouss M, Karami M. Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic Review. Clin J Obstet Gynecol. 2023; 6: 201-215.
DOI: 10.29328/journal.cjog.1001151
Copyright License: © 2023 Karami MH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Vaginal Drug Delivery Systems (VDDSs); Infections caused by microbes; Physiological barriers; In Vitro and Ex vivo models; Polymer gels
Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic Review
Mohammad Hossein Karami1*, Majid Abdouss1* and Mandana Karami2
1Department of Chemistry, Amirkabir University of Technology, P.O. Box 15875-4413, Tehran, Iran
2Polymer and Petrochemical Research Institute, P.O. Box 14975/112, Tehran, Iran
*Address for Correspondence: Mohammad Hossein Karami, Department of Chemistry, Amirkabir University of Technology, P.O. Box 15875-4413, Tehran, Iran,
Email: [email protected]
Majid Abdouss, Department of Chemistry, Amirkabir University of Technology, P.O. Box 15875-4413, Tehran, Iran, Email: [email protected]
The survey gives an in-depth examination of medicate assimilation challenges within the genital range and the improvement of vaginal medicate conveyance gadgets to overcome these challenges. It investigates the components involved in medicate discharge within the genital locale and examines commonly utilized vaginal sedate conveyance frameworks such as nanoparticles and hydrogels. The survey centers on the applications of these conveyance frameworks in controlling bacterial vaginal diseases. The plan issues related to vaginal sedate conveyance gadgets are moreover examined, highlighting the significance of considering variables such as mucoadhesion and bodily fluid porousness. The survey portrays different in vitro and ex vivo models utilized for assessing these frameworks, counting organoids and new human cervical bodily fluid. The choice of show depends on the particular objectives and characteristics of the definition. The audit emphasizes the noteworthiness of utilizing these models to pick up important bits of knowledge and make precise forecasts with respect to the execution of medicate conveyance frameworks in vivo. Moreover, grandstands progressed models utilized for other mucosal locales as a potential motivation for future models of the female regenerative framework. Generally, the audit highlights the significance of understanding organic instruments and planning compelling vaginal drug conveyance frameworks to progress sedate conveyance within the genital region. It emphasizes the require for suitable models to evaluate and anticipate the execution of these conveyance frameworks.
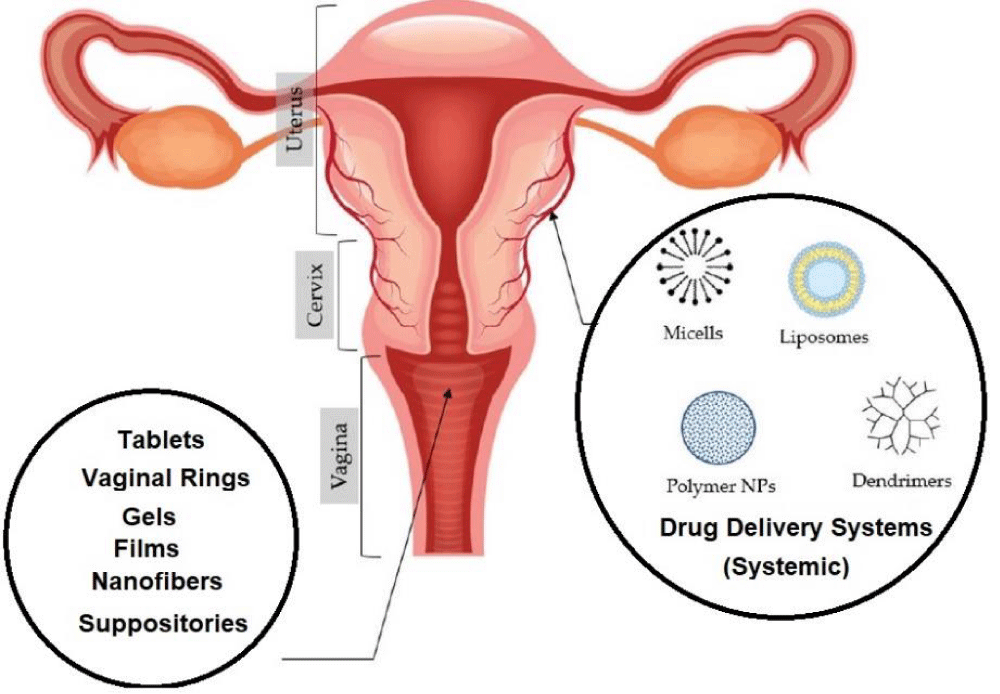
Each year, a considerable number of women experience vaginal infections caused by imbalances in the microbiome. These infections include yeast infections, bacterial vaginosis (BV), and trichomoniasis. Various diseases are responsible for these conditions (such as trichomoniasis, yeast infections, genital herpes, chlamydia, and others). Genital warts, in particular, are frequently asymptomatic in ladies but can lead to fruitlessness, pelvic torment, and release. Drawn-out contamination with genital warts also increments the hazard of uterine cancer [1,2]. Vaginal diseases caused by organisms have been the subject of expanding investigate intrigued. Vaginal tissues have special characteristics, counting an expansive surface region, tall mucosal porosity, and thick vascularization, which make them conducive to productive medicate delivery [3]. Not at all like verbal organization, drugs managed vaginally can bypass the stomach and liver, encouraging upgrading their viability. Different shapes of vaginal sedate definitions have been created (such as gels, rings, movies, pessaries, capsules, and tablets). Be that as it may, their adequacy is regularly constrained to the vaginal environment. Challenges still exist in accomplishing maintained discharge and compelling entrance of drugs into cervical liquid and the vaginal epithelium [4]. To create compelling vaginal sedate conveyance frameworks (VDDS), it is vital to get the composition of the genital zone. The genital region comprises different layers counting stratified epithelium, fibromuscular layer, lamina propria, and adventitia. It is additionally found in a locale known as the genital tract, which acts as a common boundary influencing sedate retention. Ponders have appeared that vaginal liquids can debase long-acting drugs and nanoparticles, driving to a fast end and constraining the viability of VDDS [5]. Also, the epithelial boundary shaped by epithelial cells plays a critical part in medicate conveyance to the vagina. This barrier can limit the entry of medications and impede the passage of white blood cells in their fight against bacteria [6]. Besides, there are extra boundaries inside the vagina that can influence the viability of VDDSs. These incorporate female physiological obstructions and intravaginal obstructions. The persistent generation of vaginal liquid can lead to the quick clearance of VDDSs, whereas the special vaginal microenvironment, characterized by moo pH and tall chemical levels, can improve the maintenance of VDDSs [7]. However, safe cells within the vagina can act as a defense component, ensuring against remote operators and possibly quickening the clearance of VDDSs, in this manner making a characteristic boundary. In spite of the accessibility of different dose shapes for vaginal conveyance, they frequently confront confinements in their capacity to control the destiny of dynamic compounds due to the nearness of these natural boundaries. The advancement of pharmaceutical nanotechnology and nanomedicine has given unused openings to overcome these boundaries, with the creation of nanoparticle- and hydrogel-based VDDSs that can possibly improve sedate conveyance to the vagina [8]. Sedate conveyance to the genital range faces a few challenges, counting the impact of compounds like bodily fluid, vaginal liquids, chemicals, protein inhibitors, and proteins on assimilation and sedate discharge profiles in vaginal medicate conveyance frameworks (VDSs) [9]. The normal obstructions shaped by the vaginal tissues and body ensure it from outside substances, counting drugs. Subsequently, it is significant to consider these components when creating drugs for the genital area [10]. To overcome these challenges, different VDSs have been created, such as nanoparticles and hydrogels. Nanoparticles offer focused on medicate conveyance, maintained discharge, and moved forward bioavailability, whereas hydrogels give a three-dimensional organize for controlled sedate release [11]. These VDSs have found applications in vaginal microbial control, where they can successfully convey antimicrobial operators to treat vaginal diseases caused by organisms. By defining drugs into VDSs, their adequacy can be improved, and focus on conveyance to the location of disease can be accomplished. In outline, understanding the challenges in sedate conveyance to the genital zone and utilizing VDSs like nanoparticles and hydrogels give openings for more successful medications for genital diseases and other related conditions (Figure 1) [12].
Figure 1: Depiction of vaginal physiological obstacles in vaginal medication delivery.
Vaginal infections associated with microorganisms and biological obstacles in vaginal medication administration
Structure and function of the vaginal anatomy: The vaginal canal, which expands from the cervix to the outside genitalia, measures around 9 cm in length. It is composed of folds known as rugae that frame the dividers of the genital locale. Critically, the vaginal dividers are wealthy in blood vessels, encouraging the assimilation of vaginal medicate conveyance frameworks (VDDS) [13]. This broad organize of veins permits for the quick discharge of VDDS from the vagina into the fringe circulation, bypassing the first-pass digestion system within the liver. Besides, the vaginal epithelium contains a 5-55 µm thick layer of glycoprotein gel, known as a vaginal bodily fluid, which is mindful of different bodily fluid discharges. The vagina too produces a critical sum of liquid, with ladies of regenerative age regularly producing 18-24 grams of vaginal liquid per day [14].
Vaginal infections associated with microorganisms: Millions of ladies are influenced by genital diseases caused by infections, microscopic organisms, and parasites each year. Investigate demonstrates that numerous women's wellbeing issues are credited to an excess of microorganisms (allude to Table 1) [15]. For occurrence, Chlamydia trachomatis and Neisseria gonorrhoeae, two Gram-negative microbes, are capable of chlamydial infection and gonorrhea, respectively [16]. Syphilis is caused by the bacterium Treponema pallidum. Candida albicans, a common organism, could be a major guilty party in 50% - 70% of contagious contaminations, counting vaginal candidiasis. Trichomoniasis, caused by the parasite Trichomonas vaginalis, can inundate vaginal epithelial cells, ruddy blood cells, and vaginal microscopic organisms. These contaminations can result in barrenness, pelvic torment, stomach-related issues, and an expanded chance of cancer with delayed exposure [17].
| Table 1: Disease-causing organism of female reproductive system disorder. | |||
| Vaginal disease | Infectious agent | Germ | References |
| Gonorrhea | Neisseria gonorrhea | bacteria | [10] |
| Trichomoniasis | Trichomonas vaginalis | parasite | [12] |
| Bacterial Vaginosis | Gardnerella vaginalis | bacteria | [14] |
| Syphilis | Treponema pallidum | bacteria | [15] |
| Chlamydia | Chlamydia trachomatis | bacteria | [17] |
| VC | Candida albicans | Fungus | [18] |
For occasion, gram-negative microscopic organisms, to be specific Chlamydia trachomatis and Neisseria gonorrhoeae, are dependable for diseases like chlamydia and gonorrhea [18]. In the interim, syphilis stems from the bacterium Treponema pallidum. Vaginal candidiasis (VC), a predominant contagious disease, overwhelmingly emerges from Candida albicans (C. albicans), a common yeast. C. albicans stands as one of the foremost as often as possible experienced pathogens, constituting 50% - 70% of parasitic infections [19]. Furthermore, trichomoniasis is caused by the nearness of Trichomonas vaginalis, a parasite that not as it were overwhelms ruddy blood cells and microbes within the vaginal zone but too targets vaginal epithelial cells. Be that as it may, trichomoniasis can have genuine results such as fruitlessness, pelvic torment, stomach-related issues, and an expanded hazard of creating cervical cancer over time [20].
Biological hurdles in vaginal drug administration: The vaginal bodily fluid boundary presents a noteworthy challenge in conveying drugs to the vagina. Later inquiry has uncovered that the cervix contains vaginal bodily fluid, which contains a thickness ranging from 5 μm to 55 μm [21]. This bodily fluid could be a complex blend of different components, counting vaginal epithelial cells, genital exudate, gel-forming glycoproteins, lipids, proteins, proteins, and antibodies. It overwhelmingly comprises water, making up around 90% - 95% of its composition. The vaginal bodily fluid acts as a physical obstruction, catching drugs and outside nanoparticles through a steric handle, driving to delayed indication alleviation. Furthermore, mucin filaments inside the bodily fluid ruin medicate entrance and retention within the vaginal tissues [22].
Overcoming these obstructions is significant for successful medicate conveyance to the vaginal locale. Methodologies such as adjusting sedate definitions or utilizing infiltration enhancers can upgrade medicate infiltration through the bodily fluid barrier [23]. Creating sedate conveyance frameworks that can proficiently bypass or break down the bodily fluid boundary is additionally vital for progressing sedate retention and dispersion inside the vaginal tissues. Understanding and tending to these natural boundaries are fundamental for the effective improvement of vaginal sedate conveyance systems [24]. Another significant natural figure that influences sedate conveyance to the genital region is the genital epithelium. It plays a part in diminishing medicate movement and subepithelial sedate administration [25]. Epithelial cells frame tight intersections, which constrain paracellular porousness and take-up of vaginal sedate conveyance frameworks (VDS). The absorptive efficiency of VDS is additionally impacted by the thickness of the genital epithelium. The thickness of the vaginal epithelial cell layer changes amid distinctive stages of monthly cycle, with a contrast of roughly 200-300 microns. As the thickness increments, VDS absorption decreases [26]. Subsequently, a comprehensive approach considering the complete menstrual cycle is required for compelling genital sedate conveyance. In expansion to the organic boundaries postured by the epithelial cells of the cervix and genital region, extraordinary consideration ought to be given to pregnant ladies when creating vaginal medicate conveyance frameworks. Pregnant ladies are involved in different organic changes that can affect VDS efficacy [27]. For illustration, the vaginal liquid volume in pregnant ladies is roughly 18-24 grams per day and contains compounds such as lactic corrosive, glycerol, and glucose. The nearness of glycogen within the vaginal liquid serves as a substrate for microbial and enzymatic forms that create lactic corrosive, keeping up the pH of the genital area. The pH of the vagina in premenopausal ladies regularly ranges from 4.0 to 5.0, with varieties of completely different parts of the vagina [28]. This acidic microenvironment can influence the soundness and adequacy of VDS. Besides, pregnant ladies have a changed safe framework, enlisting more resistant cells such as T cells, B cells, Langerhans cells (LC), and dendritic cells (DC) to protect the vagina from outside substances. This resistant reaction can possibly affect the work and viability of VDS. Subsequently, considering immunogenicity is additionally vital in the improvement of VDS for pregnant women [29].
Approach to addressing biological obstacles in vaginal drug delivery: The advancement of vaginal sedate conveyance frameworks (VDS) is impacted by physicochemical qualities, counting surface chemistry and molecule estimate. Analysts have already decided that the behavior of bodily fluid in VDS is unexpected upon its surface chemistry [30]. To overcome the bodily fluid boundary, researchers have created a run of VDS with mucoadhesive and mucus-permeable characteristics. One approach includes coating VDS with polymers like polyacrylic corrosive (PAA), carbomer, alginate, chitosan, and carrageenan to improve their mucoadhesive properties. These polymers can connected with bodily fluid through ionic, hydrogen holding, hydrophobic, or van der Waals intelligent, subsequently expanding the maintenance time and infiltration of VDS in bodily fluid (Figure 2) [31]. Within the setting of vaginal sedate conveyance frameworks (VDS), molecule measure, in expansion to surface chemistry, plays a vital part in their viability. By controlling the molecule estimate, the capacity of VDS to enter and be ingested through the genital boundary can be upgraded. The physical blending of VDS with cross-linked mucin strands is a vital calculation in encouraging the entrance of VDS through mucus [32]. By and large, VDS bigger than 500 nm in the estimate can enter bodily fluid, whereas bigger VDS confront more noteworthy challenges in bodily fluid entrance. Broad investigate has been conducted to make strides in the electrical properties of VDS, as their retention productivity is subordinate to their physicochemical traits. Also, variables such as the thickness, amount, composition of the vaginal epithelium, and pH esteem of the vaginal liquid in females moreover impact the retention of VDS. Be that as it may, inquiries about information on the human genitalia are still constrained. Advance examination is fundamental to way better understand the negative impacts of drugs on the genital zone and to overcome natural diseases [33].
Figure 2: Enhancing mucus transport behavior of vaginal drug delivery systems through variation in polyethylene glycol (PEG) surface density.
Commonly employed nanoparticles for overcoming biological barriers in vaginal drug delivery
Topical drugs such as tablets, capsules, and gels are commonly endorsed for vaginal medicate conveyance. In any case, their viability is frequently prevented by the boundaries displayed within the vaginal environment [34]. For a long time, progressions in nanotechnology have driven the advancement of different conveyance frameworks based on nanoparticles and hydrogels. These imaginative approaches offer promising arrangements to overcome organic obstructions. This chapter gives a presentation on regularly utilized vaginally medicate conveyance frameworks, particularly nanoparticles, and hydrogels, and explores their applications within the genital area [35].
Liposomes: Liposomes, comprised of cholesterol, phospholipids, and water are considered safe for the body as they can naturally degrade, are non-toxic, and do not elicit an immune response [36]. The aforementioned properties of liposomes make them highly advantageous for drug delivery to the vagina. Due to their ability to easily traverse the vaginal environment and remain in place for extended periods, liposomes are ideal for this purpose. Additionally, liposomes can be modified with polymers that have an affinity for bodily fluids or possess the capability to penetrate mucus. This modification enables liposomes to effectively target specific cells, even in the presence of natural barriers within the body that might impede drug delivery [37]. Later headways have driven the improvement of unused sorts of liposomes, counting altered and deformable liposomes, which can react to variables like pH, temperature, or enzymes [38]. In expansion to their fabulous grip properties, liposomes can be designed to target particular regions of the vagina, modifying their surface chemistry, morphology, and molecule estimate to enter the bodily fluid obstruction and accomplish tall medicate assimilation. Typified anti-microbials, such as clotrimazole, metronidazole, and chloramphenicol, have been effectively conveyed utilizing liposomes to treat parasitic and bacterial infections [39]. The discharge of the medicate from liposomes can be controlled by selecting the suitable liposome definition, with pH-dependent drug-release liposomes appearing to guarantee intravaginal organization due to the acidic pH of the vagina (Figure 3). Be that as it may, liposome precariousness and quick medicate discharge beneath acidic conditions have been constraining variables. To overcome these challenges, liposomes have been joined into polyacrylate gels, giving strides in steadiness and longer capacity times [40]. In vitro ponders have appeared that liposomes joined in gels show adequate soundness at low pH and have the specified consistency, hydrophilicity, and bio adhesion. These liposome gels have illustrated maintained medicate discharge and maintenance, making them promising vaginal sedate conveyance frameworks. Another approach includes the utilize of liposomes/noisesomes in combination with 2rbopol gel, which moves forward the physical soundness of liposomes. Multilayered liposomes/noisesomes have appeared tall medicate movement and soundness, with maintained discharge compared to control bunches. In creature considers, mice treated with liposomes/noisesomes containing clotrimazole shown expanded anti-inflammatory sedate levels at day 7 after administration [40].
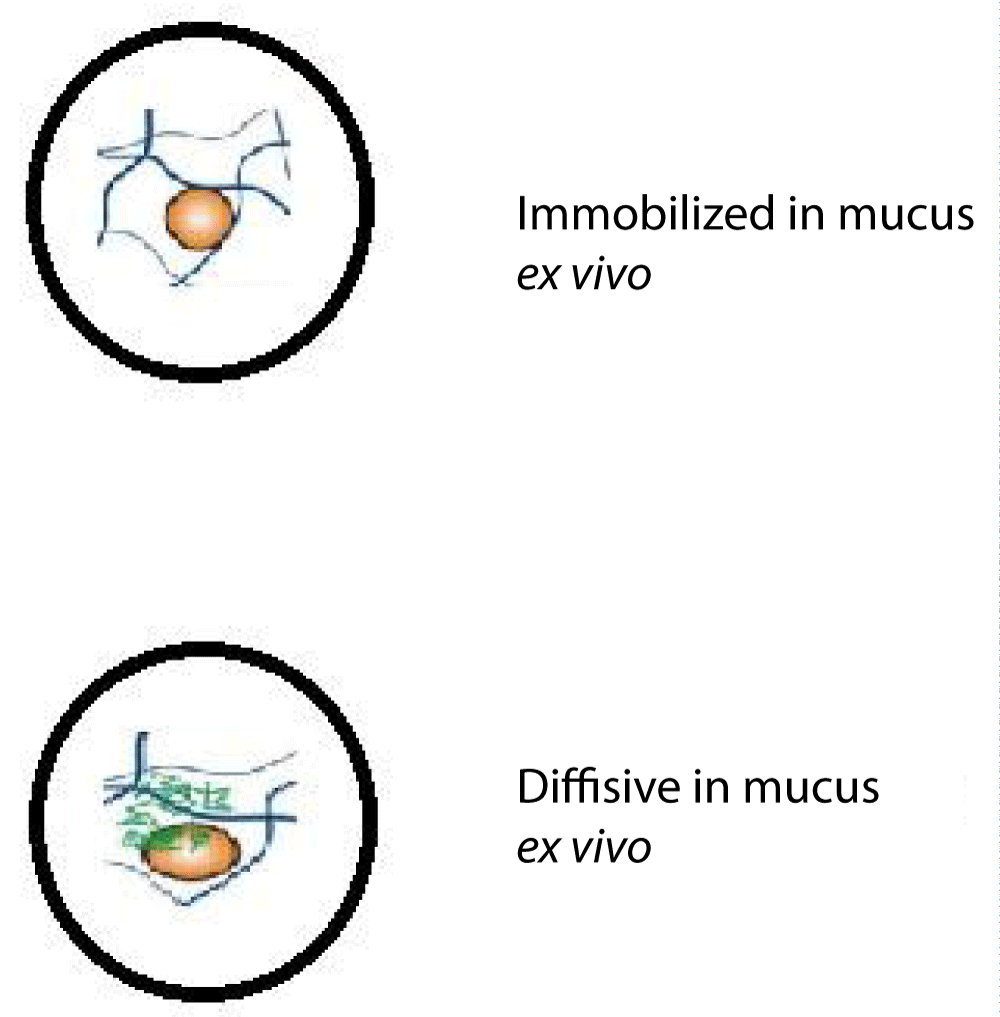
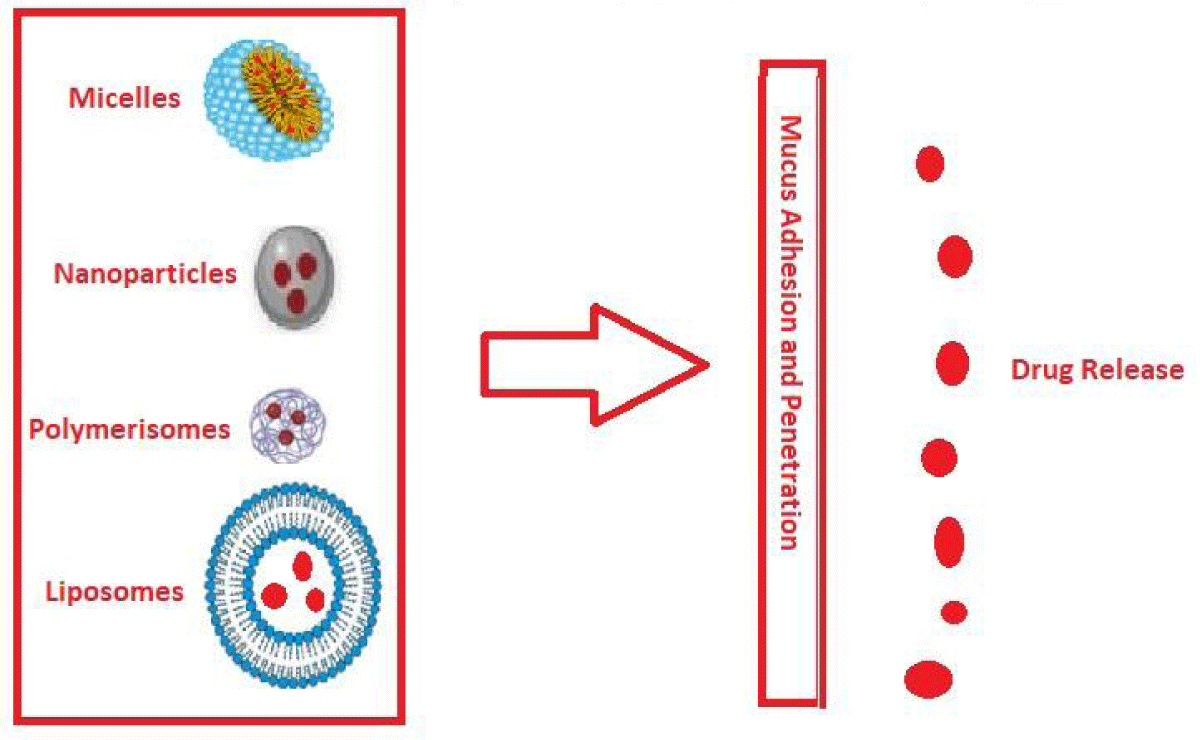
Figure 3: Evaluation of liposomes and polymer-based nanoparticles for their ability to adhere to and penetrate mucus.
The analysis of in vivo studies in the systematic review focused on investigating the effectiveness of clotrimazole-containing liposomes/noisesomes in mice. These studies provided valuable insights into how liposomes performed compared to control groups, offering implications for potential clinical applications [41]. The in vivo studies included in the review involved treating mice with clotrimazole-containing liposomes/noisesomes, while control groups received different formulations or a placebo. Various outcomes were assessed in these studies, such as reductions in microbial load, levels of inflammation, and overall therapeutic efficacy. The results of these in vivo studies consistently demonstrated that liposomes outperformed the control groups. Mice treated with clotrimazole-containing liposomes/noisesomes exhibited significant reductions in microbial load, indicating the effective control of microbe infections [42]. Additionally, the liposomes were found to significantly decrease inflammation levels in the vaginal tissues of the treated mice, highlighting their anti-inflammatory properties. These findings have important implications for potential clinical use. The utilization of liposomes as a drug delivery system for clotrimazole in the treatment of vaginal microbe infections has the potential to improve therapeutic outcomes. By delivering clotrimazole directly to the affected area, liposomes enable sustained and controlled release of the drug, ensuring prolonged exposure and potentially enhancing its efficacy. This targeted approach may lead to improved treatment outcomes and potentially reduce the risk of drug resistance [43]. Moreover, the ability of liposomes to reduce inflammation in the vaginal tissues is a promising finding. Inflammation is a common issue associated with microbe infections, and its reduction can alleviate discomfort and facilitate healing. This suggests that liposomes not only serve as effective drug carriers but also possess inherent anti-inflammatory properties that contribute to the overall therapeutic effect [44]. Based on these encouraging outcomes, the use of clotrimazole-containing liposomes/noisesomes shows potential as a clinical intervention for the treatment of vaginal microbe infections. Further research and clinical trials are necessary to validate these findings, determine optimal dosages and treatment durations, and assess potential side effects. Nevertheless, the insights gained from the in vivo studies reviewed in this systematic review provide a solid foundation for future investigations and underscore the potential clinical benefits of liposome-based drug delivery systems in managing vaginal microbe infections [45].
Researchers have been able to upgrade the properties of liposomes by controlling their physicochemical characteristics [41-43]. This has driven the advancement of modern sorts of liposomes that show progressed mucoadhesive properties and upgraded bodily fluid entrance. For occasion, Škalko-Basnet, et al. made PEGylated liposomes with a normal molecule estimate of 181 ± 8 nm and a negative charge (-13 mV) [43-45]. These liposomes illustrated way better infiltration through bodily fluid and more profound epithelial layers compared to control liposomes. In another ponder, Hanan Refai, et al. coated liposomes with chitosan for the vaginal conveyance of sildenafil citrate. The chitosan-coated liposomes displayed drawn-out discharge and moved forward the porousness of sildenafil compared to uncoated liposomes, driving improved retention of sildenafil within the vaginal region [43]. Later progressions in nanotechnology have driven the improvement of stretchable liposomes, which offer modern conceivable outcomes for vaginal medicate delivery [44]. Dong Chunjing, et al. have detailed the potential utilize of deformable liposomes as a novel instrument for this reason. Essentially, Rofida Allash, et al. conducted a think about the co-treatment of genital candidiasis utilizing propylene glycol-loaded fenticonazole nitrate (FN) with deformable liposomes (FDL) [45]. The FDL detailing illustrated adaptable coating, with a molecule measure of 185 ± 19 nm and a negative charge of -53 ± 2.7 mV. It moreover showed tall deformability of 92% ± 5.6% phospholipid/min. The nearness of propylene glycol and the hydrophilicity of the phospholipid bilayer permitted FDL to proficiently typify FN, with a sedate stacking effectiveness of 78% ± 2.14%. Furthermore, propylene glycol improved the penetrability of FN through the vaginal mucosa [46]. These deformable liposomes encouraged the entrance of FN into the genital mucosa, empowering better measurements of the medicate to be kept up within the genital tissue while avoiding systemic absorption. This moved-forward conveyance framework appears to guarantee upgrading the adequacy of treatment within the genital area [47].
Biocompatible nanocarriers for vaginal therapy: Characteristic polymers offer a few points of interest for utilize in pharmaceuticals, such as their biocompatibility, reasonableness, accessibility of crude materials, controlled discharge capabilities, and moo toxicity [48]. Polysaccharides determined from plants and proteins like gluten and zein are broadly examined and utilized in pharmaceutical details. In any case, there's constrained data, particularly on the utilize of normal polymeric nanoparticles for vaginal medicate conveyance. Two promising polysaccharides, chitosan, and alginate, have appeared potential as medicate carriers for vaginal medications. They have mucoadhesive properties, antimicrobial movement, and the capacity to regulate drug discharge within the vaginal mucosa [49]. Chitosan, in specific, has been broadly considered for its capacity to move forward fibroblast multiplication, display antioxidant properties, and upgrade medicate retention in vaginal tissues [50]. Alginate-based nanoparticles, determined from green growth and created through biotechnological strategies, have moreover been investigated for vaginal medicate conveyance. Proteins, such as zein, determined from plants, have also been considered for the advancement of characteristic polymeric nanoparticles. Zein shows bioadhesivity, biocompatibility, and anticancer properties. When combined with chitosan, zein can upgrade medicate entrance through the bodily fluid layers of the vagina. Generally, normal polymers within the shape of nanoparticles appear to guarantee vaginal medications due to their biocompatibility, moo harmfulness, and capacity to be associated with the vaginal mucosa. In any case, advance investigate is required to investigate their viability, security, and optimization for medicate conveyance by means of the vaginal route (Table 2) [51].
| Table 2: Utilization of natural polymers in the formulation of nanoparticles for vaginal mucosal applications. | ||||
| Biopolymer | Formulation | Drug encapsulation | Therapeutic approach | References |
| Chitosan | Ionotropic gelation |
Ascorbic acid | Cervical Cancer | [15] |
| Gelatin | Desolvation method |
Tenofovir | Sexual transmission of HIV in women | [25] |
| Chitosan | Ionotropic gelation |
Insulin | Peptide delivery system | [31] |
| Ovomucin | Nano-precipitation | Ciprofloxacin Riboflavin |
Unreported | [40] |
| Alginate | Reverse emulsification |
Silver saccharinate |
Inhibiting HSV-2 ( Herpesvirus type 2)and Neisseria gonorrhoeae | [42] |
| Chitosan ascorbate |
Ionotropic gelation |
Afamoxicillin trihydrate |
Atrophic Vaginitis | [45] |
The field of vaginal drug delivery has witnessed significant advancements in liposome technology, particularly in the realm of modified and deformable liposomes [38-41]. These innovations have been developed to address the challenges encountered with conventional liposomes and present numerous benefits. Modified liposomes are designed with specific surface properties that enhance their interaction with the vaginal mucosa [42-45]. By altering the surface charge, size, or composition of liposomes, their uptake and retention in the vaginal tissue can be greatly improved. Deformable liposomes, on the other hand, possess the unique ability to change shape and adapt to the biological barriers encountered during vaginal drug delivery [46-48]. This adaptability enables them to penetrate the vaginal epithelium more effectively and reach the desired site. These advancements in liposome technology offer several advantages over traditional liposomes. Firstly, they enhance the bioavailability of drugs by increasing their retention and absorption in the vaginal tissue [49]. Moreover, modified and deformable liposomes can enhance the stability and release profile of encapsulated drugs, ensuring controlled and sustained delivery. Additionally, these innovative liposomal formulations provide improved drug targeting and reduced systemic exposure, minimizing potential adverse effects. They also allow for the encapsulation of a wide range of drugs, including both hydrophilic and hydrophobic compounds, expanding the therapeutic possibilities for vaginal drug delivery [50]. In conclusion, recent progress in modified and deformable liposomes has revolutionized vaginal drug delivery by overcoming the limitations associated with traditional liposomes. These advancements offer improved drug bioavailability, enhanced stability, targeted delivery, and a broader range of encapsulation options. They hold great promise in enhancing the effectiveness and safety of drug therapies in the vaginal region [51].
Nanogel systems: Hydrogels have profitable physical, chemical, and natural properties that make them appealing as potential frameworks for vaginal sedate conveyance (VDDSs). Their remarkable mucoadhesive characteristics and moo harmfulness render hydrogels exceedingly successful in conveying drugs to the vaginal region [52]. By consolidating pH-sensitive and thermoresponsive properties, hydrogels empower exact and controlled discharge of vaginal drugs. Be that as it may, their expansive measure limits their capacity to enter bodily fluid effectively [53]. To overcome this restriction, analysts have created different composite frameworks combining nanoparticles with hydrogels. For illustration, Lars Eckmann and colleagues created a thermoresponsive hydrogel implanted with nanoparticles to convey auranofin. Akpa and colleagues portrayed mucoadhesive hydrogels with adjusted surfaces for conveying miconazole nitrate (MN), which decreases bothering, upgrades entrance, and drags out localized vaginal sedate discharge for successful treatment. So also, gelatin-carbohydrate hydrogels stacked with nanoparticles were utilized to move forward the adequacy of metronidazole (MZ) in treating vaginal contaminations, especially Candida contaminations [54]. These composite frameworks take advantage of the amazing mucoadhesion of hydrogels and empower proficient medicate stacking in vaginal bodily fluid. Analysts have found that the expansion of nanoparticles encourages more profound infiltration through bodily fluid, overcoming organic boundaries and coming about in higher antimicrobial action against microbes such as Escherichia coli (E. coli) and Bacillus subtilis [55]. Temperature-sensitive and pH-sensitive hydrogels have been broadly considered for controlling vaginal contaminations. For occurrence, Castillo-Castro, et al. created thermosensitive poly(methyl vinyl ether-alt-maleic anhydride) (PVMEMA) hydrogels that drag out sedate home within the vagina, improving their restorative viability against bacterial vaginosis. Janat-Amsbury, et al. made a thermosensitive mucoadhesive polymer-based progesterone (P4) gel (GC-P4) for vaginal application, which may possibly stop the movement of endometrial hyperplasia [56]. Responsive hydrogels, not at all like routine hydrogels, offer noteworthy points of interest in terms of controlled medicate discharge and are picking up ubiquity in vaginal medicate conveyance. The utilize of composite frameworks comprising nanoparticles and hydrogels permits moved forward bodily fluid infiltration, improving sedate maintenance, entrance, assimilation, and restorative impacts for vaginal infections [57].
Nanoscale inorganic particles: Inorganic nanoparticles have been utilized for their antibacterial properties for a long time. Later thinks have uncovered that distinctive sorts of inorganic nanoparticles can actuate harm to microbial cells through oxidative push, protein inactivation, or film disruption [58]. These properties contribute to their tall antimicrobial movement, making them profitable as antimicrobial operators. In expansion, inorganic nanoparticles illustrate fabulous steadiness in cruel situations, counting moo pH levels, tall chemical concentrations, and tall protein substances within the vagina. As a result, they hold critical potential for treating genital contaminations. Additionally, nanoscale inorganic nanoparticles have demonstrated successful in entering vaginal mucus [59]. Silver-based nanoparticles, particularly silver nanoparticles (Ag NPs), are broadly utilized for their antibacterial properties against both Gram-positive and Gram-negative microbes. The antibacterial action of Ag NPs closely takes after that of silver particles. For occurrence, Chen, et al. detailed that Ag0 NPs can disturb bacterial cell judgment, leading to cell death [60]. Besides, Hua Wei, et al. highlighted that the era of intracellular responsive oxygen species (ROS) actuated by Ag NPs is considered the essential instrument behind their cytotoxicity [61]. Additionally, several studies have demonstrated that silver-based nanoparticles inhibit DNA replication by binding to the sulfhydryl groups present in bacterial cells. For example, Ag2O nanoparticles bind to bacterial DNA, preventing bacterial replication and causing DNA damage, ultimately resulting in bacterial death [62]. Gold-based nanoparticles, particularly gold nanoparticles (Au-NPs), have developed as promising candidates for combating microbial contaminations. Xie J, et al. found that Au NPs can upgrade intracellular receptive oxygen species (ROS) generation, subsequently moving forward their antibacterial activity [63]. They observed that the antibacterial impact was subordinate to the measure of the nanoparticles, with ultra-small gold nanoparticles showing more prominent antibacterial efficacy [64]. These small-sized Au NPs have the capacity to create ROS inside bacterial cells, driving bacterial cell passing. These interesting properties of Au NPs have the potential to improve safe reactions, especially in photothermal/photodynamic treatment. For occurrence, Jiang's gather created a bacteria-derived AuNP get-together framework for photothermal antibacterial treatment. These nanoparticles can total on bacterial cells, and through the bioorthogonal cycloaddition of vancomycin, their photokilling movement against Gram-positive microbes is upgraded. In addition, the surface chemistry of Au NPs can be altered by consolidating antimicrobial specialists such as antimicrobial peptides, chitosan, and quaternary ammonium bunches, which have noteworthy applications in antimicrobial therapy [65]. Optimizing the utilitarian bunches on the surface of gold nanoparticles utilizing cationic and hydrophobic ligands may be a promising procedure to overcome multidrug resistance (MDR) in microscopic organisms and combat illnesses effectively [66]. Later investigations have appeared that different sorts of inorganic nanoparticles, counting zinc-based (such as ZnO), nickel-based, copper-based, and palladium-based nanoparticles, have antibacterial and antifungal properties [67]. Copper nanoparticles, in specific, have been found to show antimicrobial movement against microscopic organisms like Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and certain strains of fungi [68]. Moreover, the utilization of metal half-breed nanoparticles has appeared to guarantee improving antimicrobial impacts. These crossovers have predominant catalytic execution in producing responsive oxygen species (ROS) and illustrate moved forward photothermal or electronic change productivity. For occasion, the application of Fe3O4-Ag cross-breed nanoparticles has been found to result in bigger E. coli cells compared to utilizing AgNPs alone [69]. In spite of the noteworthy antibacterial properties of inorganic nanoparticles, their utilization in clinical settings is right now constrained due to concerns around their biosafety. Subsequently, assistance inquiries are essential to diminish the toxicity related to inorganic nanoparticles and guarantee their security for clinical applications [70].
In vitro models aid in the development and optimization of vaginal drug delivery systems
Mimics vaginal environment: Vaginal medicines can affect the stability and release of drugs in the cervicovaginal fluid. To assess these possible intuitions, research facility analysts often use simulated vaginal fluid or purified mucin solutions [71]. The SVF is made up of information about the chemical composition of fluids in the vagina. This information focuses on properties that affect how well products applied to the vagina work [72]. pH refers to how acidic or alkaline a substance is, while osmolarity refers to the concentration of solutes in a solution. The SVF formulas usually include salt that is balanced to pH 4. 2 They also have bovine serum, egg whites, corrosive ingredients, glycerol, urea, and glucose. However, they do not contain mucin. Other definitions of SVF with additional supplements and higher pH have been suggested to study the growth of bacteria. Reenacted cervical bodily fluid has also been suggested but not widely used to study drug delivery systems [73].
According to Araujo, et al. a gel-like structure was created using a combination of oleic acid, cholesterol, ethoxylated and propoxylated cetyl alcohol (a special type of alcohol), and poloxamer 407. When mixed with a certain type of protein, the gel turned from a liquid to a more solid texture, showing that it can stick to mucin cuts in a stretched-out form, according to tests in a lab [74]. These gel samples containing SVF also showed calming release patterns in laboratory experiments. In another study by Huang and others, they made special fibers using cellulose and a chemical called acetic acid phthalate. They added a medicine (TMC 125 or tenofovir disoproxil fumarate) that fights against HIV to these fibers [75]. They did this to see if these fibers could be used in the vagina to treat HIV. This fiber is designed to use the pH-dependent ability of CAP to dissolve and release the drug rapidly when it comes into contact with semen. The research analysts discovered that the strands of a substance called CAP stayed the same when exposed to the natural pH level of the vagina, which is around 4. 2 However, when semen, which has a higher pH level of 7. 4-84, came into contact with the strands, it caused them to change and release the medication immediately [76]. Changing the amount of SVF mixed with semen showed that the drug-filled filaments broke apart within 8-90 seconds, depending on how much semen was present. Moreover, when the CAP filaments mixed with tenofovir disoproxil fumarate were exposed to HIV-1 particles in a liquid at a pH of 7. 4 for 1 hour, and then added to CD4+ TZMbl cells, a strong infection prevention effect was observed, which increased with higher concentrations. Mucus-filled SVF was used as a cervicovaginal fluid (CVM) model to study interactions with drug-filled nanoparticles [77]. Neves and others. The researchers in this study focused on developing small particles called polycaprolactone (PCL) and coating them with the antiviral medicine dapivirine. To create variations, they modified the outer surface of the particles using different substances such as poloxamer 338 NF, PEO (a neutral compound), cetyltrimethylammonium bromide (a positively charged compound), or sodium lauryl sulfate (a negatively charged compound) [78]. To mimic the stretchiness of CVM, they added a small amount (1.5%) of a modified version of pig stomach fluid called sort II to create a new substance called SVF. Rheological studies found that mucin-containing seminal vesicle fluid (SVF) had a lower thickness compared to non-ovulatory cervical mucus (CVM), but a thickness profile more similar to ovulatory CVM [79]. As expected, because mucin is negatively charged, the CTAB-PCL particles became larger when mixed with SVF containing mucin at pH 4. 2 or pH 7. 0 The study observed that nanoparticles labeled with fluorescent markers did not move much when placed in SVF, regardless of the pH. This suggests that CTAB-PCL nanoparticles stick very well to the SVF [80]. In simpler terms, PEO-PCL and SLS-PCL nanoparticles were found to be more versatile and diffuse more easily in the SVF at pH 7. 0 compared to pH 4. 2 This may be because the ionization state of mucin changes at different pH levels. Generally, the SLS-PCL nanoparticles moved more than the CTAB-PCL nanoparticles in a substance called SVF, at both pH 4. 2 and 70 The SLS-PCL nanoparticles moved 6 times more at pH 4. 2 and 36 times more at pH 7. 0 compared to the CTAB-PCL nanoparticles [81]. Used SVF to study a dried sticky framework filled with lipoplexes designed to release siRNA when the vagina becomes wet again [82]. Lipoplexes are covered with smaller parts of polyethylene glycol (PEG) to reduce sticking together with bodily fluids. DSPE-PEG2000 was chosen as the best option because it allows the lipoplexes to move easily and stay stable in SVF at pH 6, which contains 1.5% of a type of pig mucus called sort 3 porcine mucus. Using hydroxyethylcellulose and PEG wipes helps define lipoplexes, which have a better grip on somewhat hydrated mucin plates compared to other gels and creams available in the market [83]. The researchers in this study demonstrated a method for cleaning and moisturizing lipoplexes. They examined the degradation properties of vaginal tablets containing antifungal medicine using simulated vaginal fluid (SVF) without mucin. To evaluate the effectiveness of the medication, the tablets were exposed to a controlled flow of fluid that mimicked natural vaginal fluid production. The study found that by incorporating cysteine into poly(acrylic acid) (PAA), the stickiness of PAA to bodily fluids was improved. This modification also enhanced water absorption and optimized drug release in laboratory tests, outperforming unmodified PAA [84].
Tissue culture models: These models were mainly used to study various natural aspects like viral diseases, inflammation, and communication between host cells and microbiota. Regardless, they can also measure particular aspects of vaginal medication information, particularly body flexibility [85]. For example, Gali and his colleagues. Researchers used cultivated uterine and cervical cells in a special setup to study how well they survived and how they reacted when exposed to different types of ingredients in medicines and substances that kill microbes [86]. It seems that many substances used in vaginal gels can make it harder for cells to work properly and can affect the outer layer of cells. This emphasizes the importance of using in vitro screening methods to detect potential safety issues in pharmaceutical ingredients. One such method is the MatTek EpiVaginal 3D tissue, which consists of human cells that resemble vaginal tissue. These tissues are commonly used to test whether intravaginal drug delivery products are harmful. These studies have used electrospun nanofibers to prevent both HIV-1 and HSV-2 infections, polyurethane vaginal rings for slow release of dapivirin, and gel formulations to prevent C [87]. Infections The EpiVaginal show is used to test how well drugs are absorbed in the body when there is no fluid layer and regular clearance mechanism. This has been studied in relation to trachomatis and HIV infection. Some studies suggest that the health of vaginal tissue and its ability to react to infections can be influenced by different types of bacteria and their byproducts. This information is very important when it comes to defining medicine that is delivered inside the vagina for different uses, like treating bacterial vaginosis [88]. A new test developed by SkinEthic allows researchers to study and understand vaginal candidiasis better. It involves examining interactions between pathogens and cells in the vaginal lining, as well as immune responses [89]. We can study how our body's natural defenses and treatments for fungal infections affect diseases in the mucosa by adding resilient cells and tiny organisms to the models. In addition to the regular tissue culture system, researchers also created a model of human cells that imitate vaginal tissue better. Hhelm and colleagues. The researchers made artificial human vaginal tissues using special cells and equipment [90]. These 3D images showed specific details of small structures on the surface of cells, like tiny projections and folds. It also showed a stronger staining of certain connections between cells and proteins, and an increased presence of a specific type of mucin attached to the cells [91]. The study found that when exposed to nonoxynol-9, the response of cells in the vaginal tissue was similar to that of human cervical tissue samples. The 3D model used in the study showed potential in evaluating the safety of vaginal products. 3D cell totals are also important for studying how cells interact with microorganisms in tissues. Rewrite this text in simpler words: Radke and his colleagues [92]. It seemed like the problems observed in 2D cell communities were better copied in his 3D presentation. Furthermore, Doerflinger and his colleagues conducted a study where they combined 3D cell cultures with bacteria commonly found in the vagina, including Lactobacillus and bacteria associated with bacterial vaginosis (BV). They then quantified the interactions between the cells and the bacteria. Likewise, a comparable study was carried out by Radtke, et al. [93]. The researcher successfully developed a 3D model of the cervical epithelium by utilizing A2EN cells. These cells were cultivated on collagen-coated dextran microcarrier dots within a spinning bioreactor. Even though cells need a dissolvable bodily fluid layer, they showed higher expression of certain substances called mucins, especially MUC1, which is found in the cell membrane, compared to normal cell layers. I will simplify the text for you: When 3D models of cervical cells were shown to microorganisms, it caused an increase in the production of certain proteins and chemicals found in human cervical fluids [94]. Changing the way these mucins work or releasing them may have an important role in how pathogens interact with our bodies. Additional exams looked at the connection between distinct types of microbes in the vagina, medicines that fight against microbes, and different types of infections [95]. These exams showed how important it is to think about the microbes in the vagina when testing new treatments that are meant for specific microbe environments. This study shows that studying the bacteria in the vagina can help us understand how it affects the pH of bodily fluids and other substances that reduce inflammation [96]. This information could be useful in developing new ways to deliver medicine. It seems that when cervical cells are treated with a substance called cervicovaginal liquid (CVF) with a low pH and solid consistency, their function improves. This is especially true when exposed to another substance called polyinosinic-polycytidylic acid (PIC) which activates a certain receptor called TLR3. This suggests that CVF has a protective effect on cervical cells [97]. When cells treated with CVF were mixed with L-lactate, they also produced fewer cytokines like IL-6 and TNFα in response to PIC. This means that there could be communication between cells in the vagina, including the cells that line the vagina, immune cells, bacteria, and fluid in the vagina. These studies provide valuable information about improving artificial models that can better mimic the setting of the vagina. By examining different things like cells, bacteria, and fluids in the vagina, researchers can better understand its workings [98].
Preclinical models for assessing vaginal drug delivery systems (Ex vivo models)
Tissue models: A method to test drugs in the vagina is to use tissue tests outside of the body. It is important to compare animals and humans when studying reproductive organs. However, animal tissue is often used because it is easier to get [99]. These differences include differences in body structure, the microorganisms in the vagina, the surrounding environment, how hormonal cycles affect cells and tissue, gland secretions, and when hormonal cycles occur. New research suggests that the properties of cervical fluid in female mice during their reproductive cycle are similar to the properties of cervical fluid in women. In mice, the estrous cycle happens very fast. The vaginal surface and thickness change in four stages that only last 4-5 days. Hormone replacement therapy has been found to greatly impact the structure and properties of mouse CVM [100]. In a study, rats were given fluorescently labeled nanoparticles in their vagina. Some rats were treated with estradiol or progesterone to cause changes similar to high estrogen or high progesterone levels during the estrous cycle [101]. The tissue that was cut is then seen using a machine that can detect changes in the CVM layer of genital tissue. The study discovered that very small particles called mucoinert nanoparticles, measuring only 100 nm, were greatly affected by the movement within the CVM of mice that had been given progesterone beforehand. In experiments, small particles called mucoinert were spread throughout the CVM, covering the genital tissue of mice that were either treated with estradiol or were in their regular reproductive cycle [102]. Differences in the way rodents move their reproductive organs outside of the body are connected to how these organs function inside the body. These findings show that using tissue taken from mice and studied outside of the body (ex vivo) can be helpful in evaluating drugs or treatments for the reproductive system. When we analyze and show results, it's important to remember the restrictions and differences between animal models and human FRT. Pig and cow genital tissues from slaughterhouses are often used as ex vivo tissues to test drugs in the reproductive system. In their study, the researchers utilized various methods and shapes to evaluate the adhesive properties of different samples of tissue [103]. To give an example, Jalil and the co-authors. The researchers studied the ability of gellan gum-based vaginal layers with microbicide to stick to confined porcine genital tissues [104]. The inner lining of the vagina was cleaned with a substance that imitates vaginal fluid, which has a pH of 4. 2 Then, it was exposed to different materials for 10 minutes so that it could stick to the surface of the lining. The tissues were cleaned using a pump, and the collected fabric was examined to see how well it sticks to mucus. Movies that have been made with adjusted gellan gum and thiol buildups stick better compared to movies made with unmodified gellan gum [105].
Various methods and shapes were used to measure how well different substances stick to tissues. We studied how well a fungicide called gellan gum sticks to the genital film of pig genital tissue [106]. The lining inside was cleaned with imitation vaginal fluid (SVF) at pH 4. 2 and then exposed to different polymers for 10 minutes to allow water on the surface. In a different plan, researchers looked at small particles with liposomes that float in the air and micro-sized particles with nystatin loaded inside them. They tested these particles by keeping two pieces of pig genital tissue on a table made of wood [107]. In this particular study, the researchers utilized tissue from cow reproductive organs to examine the adhesive capabilities of films composed of metronidazole hyaluronic acid. The objective was to evaluate the effectiveness of these films in adhering to surfaces. The main part of the film was observed to measure the time it took to spread, and the creators discovered that their film stayed in the walls of the vagina in both directions of movement. These pictures show how pig and cow tissues are used in different tests to see how well different products stick to them in experiments outside of the body [108]. These models provide a better understanding of how specialists and tissues interact. This understanding can help improve the availability of medicine at the development site.
Ex vivo means using genital tissues from different animals, like rabbits, guinea pigs, cattle, sheep, pigs, and non-human primates, to study how drugs and molecules can get into the body. To determine if pig tissue can be used as a good model for how drugs enter human tissue [109]. They compared tissue from pigs' genitals to tissue taken from older women after they had their uterus removed. The tissue was placed in a drain to remove the cells and then exposed to a special solution containing tritium for a whole day [110]. In simpler words, the harmful glues and the way tissues are handled during collection can also affect the flow. Surprisingly, the study discovered that even though the tiny particles were mixed with cement in a lab version of vaginal fluid, they actually became more absorbent to pig vaginal tissue [111]. The reason for the increased porosity might be that the washed pig tissues do not have a layer of fluid, and the nanoparticles are toxic. Studies done on vaginal tissue outside of the body have also shown that medicinal substances stay in the genital area instead of going through the tissue [112]. Ex vivo tissue tests are used to check if topical products are toxic. Comparing different cell lines from the cervix, vagina, and uterus with isolated pig vaginal tissue to establish a reliable method for evaluating vaginal semisolids. To comprehend the potentially harmful effects of various vaginal oils, the researchers employed polarized human cervical biopsies. The study revealed that high osmolarity is linked to heightened toxicity. The significance of testing tissue samples outside of the body is emphasized as it allows for the assessment of the safety of vaginal products [113]. This is especially important when looking at things like high osmolarity and the use of certain materials in the product.
Human mucus models: Different ways are used to collect bodily fluids and mucus from the female reproductive system. This plan collects fluid from the lining of the body, which is important for being mature [114]. Alternatively, central and proximal CMPs can be gathered during pregnancy using a thin tube. Another way to collect fabric to measure dissolvable markers in the vagina is cervicovaginal lavage (CVL) [115]. This text talks about using special cups to collect a fluid called cervicovaginal fluid (CVM) in a natural and safe way. Ponder members can get their own tests by easily putting in and taking out the menstrual cup, which is then spun around to collect safe body fluid. The menstrual container is also used to collect cytokine and antibody samples by soaking for 1-2 hours [116]. Knowing the size and openness of pores in bodily fluids is important for designing and improving drug delivery systems. These systems can enter bodily fluids and deliver helpful medicine to tissue. The researchers used different methods to measure the size of the pores in the cervix [117]. One plan involves gauging the difference between proteins, microbes, and infections using special dyes. These substances can be found in cervical fluid and saltwater solution. The items spread at similar rates in cervical fluid and saline, indicating that they were small enough to be influenced by the liquid inside the fluid pores [118]. However, later studies have shown that certain particles like HSV and HIV can be found in bodily fluids and can be spread through contact. To get a better idea of how well the pore estimate works, we use tiny particles that don't stick together called bodily fluid entering particles (MPPs). These MPPs have sizes that range from 100 nm to 1 µm and are used to measure how liquid flows and to estimate the size of pores in milk [119].
In research, it has been found that factors like pH level, the types of bacteria in the vagina, and the risk of premature birth can affect the structure and properties of the cervix [120]. To explain further, when examining the cervical fluid of women at risk of premature delivery, there are more mucin strands present. This increases the ability of the granules to pass through [121]. This means that the hole size in the blood clot might be smaller in this situation. The information from these studies helps us understand how bodily fluids work and also gives important ideas for making better ways to deliver medicine [122]. This shows the need to create tiny particles or substances that can go into the fluid in the cervix and bring medicines or genetic material to the cells on its surface. In simpler terms, this sentence is saying that it is important to optimize the delivery of medicine to improve how it moves through the body and how effective it is. It also shows how different ingredients can affect how bodily fluids function [123,124].
Drug delivery systems (DDS) have the potential to effectively treat different genital infections, such as genital warts caused by various pathogens. By improving the way vaginal drug delivery systems work, they can stick to and go inside the genital area, making drugs work better and be absorbed more easily. In simpler words: Delivering drugs directly to a specific area in the body helps the medicine work better and reduces problems in other areas of the body. It is very important to understand these problems in order to create better ways to give medicine for infections in the vagina. Using medicine through the vagina is usually the best way to treat infections and problems in the female reproductive system. It helps the medicine reach the right places while reducing any unwanted effects. To make vaginal drug delivery more effective and compatible with the body, we should use suitable model systems to show how it can be used in real medical situations. When creating and choosing these models, it's important to think about the structure of the female reproductive tract and the difficulties that come with mucus, tissues, hormones, and the bacteria found in the vagina. These things can greatly affect how well drugs are delivered into the vagina and how they work. The way we predict outcomes of lab tests will get better as we develop cell cultures that imitate the layers of cells in the body, including mucus, bacteria in the vagina, and the immune system's reaction. These new models can help us understand how drugs placed in the vagina work, which can help us design better treatments that work more effectively.
Author contributions: Mohammad Hossein Karami: Formal analysis, Data Curation Investigation, Resources, Writing – Original Draft, Methodology, Conceptualization, Validation, Resources, Writing- Review & Editing, Visualization, Project administration, Funding acquisition. Majid Abdouss: Conceptualization, Validation, Editing, Supervision. Mandana Karami: Editing.
Ethics approval: This is a review study.
Consent to participate: Informed consent was obtained from all individual participants included in the study.
Consent to publish: The authors affirm that the review research provided informed consent for publication.
- Antaris AL, Chen H, Cheng K, Sun Y, Hong G, Qu C, Diao S, Deng Z, Hu X, Zhang B, Zhang X, Yaghi OK, Alamparambil ZR, Hong X, Cheng Z, Dai H. A small-molecule dye for NIR-II imaging. Nat Mater. 2016 Feb;15(2):235-42. doi: 10.1038/nmat4476. Epub 2015 Nov 23. PMID: 26595119.
- Lee JS, Youn YH, Kwon IK, Ko NR. Recent advances in quantum dots for biomedical applications. Journal of Pharmaceutical Investigation. 2018; 48(2): 209-214.
- Biswas MC, Islam MT, Nandy PK, Hossain MM. Graphene quantum dots (GQDs) for bioimaging and drug delivery applications: A review. ACS Materials Letters. 2021; 3(6): 889-911.
- Vedhanayagam M, Raja IS, Molkenova A, Atabaev TS, Sreeram KJ, Han DW. Carbon Dots-Mediated Fluorescent Scaffolds: Recent Trends in Image-Guided Tissue Engineering Applications. Int J Mol Sci. 2021 May 20;22(10):5378. doi: 10.3390/ijms22105378. PMID: 34065357; PMCID: PMC8190637.
- Facure MHM, Schneider R, Mercante LA, Correa DS. A review on graphene quantum dots and their nanocomposites: from laboratory synthesis towards agricultural and environmental applications. Environmental Science Nano. 2020; 7(12): 3710-3734.
- Chen W, Lv G, Hu W, Li D, Chen S, Dai Z. Synthesis and applications of graphene quantum dots: a review. Nanotechnology Reviews. 2018; 7(2): 157-185.
- Chen F, Gao W, Qiu X, Zhang H, Liu L, Liao P, Fu W, Luo Y. Graphene quantum dots in biomedical applications: Recent advances and future challenges. Frontiers in Laboratory Medicine. 2017; 1(4): 192-199.
- Nurunnabi M, Khatun Z, Huh KM, Park SY, Lee DY, Cho KJ, Lee YK. in vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano. 2013 Aug 27;7(8):6858-67. doi: 10.1021/nn402043c. Epub 2013 Jul 9. PMID: 23829293.
- Qiu J, Zhang R, Li J, Sang Y, Tang W, Rivera Gil P, Liu H. Fluorescent graphene quantum dots as traceable, pH-sensitive drug delivery systems. Int J Nanomedicine. 2015 Oct 28;10:6709-24. doi: 10.2147/IJN.S91864. PMID: 26604747; PMCID: PMC4630193.
- Raja IS, Molkenova A, Kang MS, Lee SH, Lee JE, Kim B, Han DW, Atabaev TS. Differential Toxicity of Graphene Family Nanomaterials Concerning Morphology. Adv Exp Med Biol. 2022;1351:23-39. doi: 10.1007/978-981-16-4923-3_2. PMID: 35175610.
- Tabish TA, Scotton CJ, Ferguson DCJ, Lin L, der Veen AV, Lowry S, Ali M, Jabeen F, Ali M, Winyard PG, Zhang S. Biocompatibility and toxicity of graphene quantum dots for potential application in photodynamic therapy. Nanomedicine (Lond). 2018 Aug 1;13(15):1923-1937. doi: 10.2217/nnm-2018-0018. Epub 2018 Aug 20. PMID: 30124363.
- Li P, Xu T, Wu S, Lei L, He D. Chronic exposure to graphene-based nanomaterials induces behavioral deficits and neural damage in Caenorhabditis elegans. J Appl Toxicol. 2017 Oct;37(10):1140-1150. doi: 10.1002/jat.3468. Epub 2017 Apr 18. PMID: 28418071.
- Nigam P , Waghmode S , Louis M , Wangnoo S , Chavan P , Sarkar D . Graphene quantum dots conjugated albumin nanoparticles for targeted drug delivery and imaging of pancreatic cancer. J Mater Chem B. 2014 Jun 7;2(21):3190-3195. doi: 10.1039/c4tb00015c. Epub 2014 Apr 28. PMID: 32261580.
- Wang S, Cole IS, Li Q. The toxicity of graphene quantum dots. RSC Advances. 2016; 6(92): 89867-89878.
- Tajik S, Dourandish Z, Zhang K, Beitollahi H, Le QV, Jang HW, Shokouhimehr M. Carbon and graphene quantum dots: a review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020 Apr 20;10(26):15406-15429. doi: 10.1039/d0ra00799d. PMID: 35495425; PMCID: PMC9052380.
- Henna TK, Pramod K. Graphene quantum dots redefine nanobiomedicine. Mater Sci Eng C Mater Biol Appl. 2020 May;110:110651. doi: 10.1016/j.msec.2020.110651. Epub 2020 Jan 8. PMID: 32204078.
- Masteri-Farahani M, Ghorbani F, Mosleh N. Boric acid modified S and N co-doped graphene quantum dots as simple and inexpensive turn-on fluorescent nanosensor for quantification of glucose. Spectrochim Acta A Mol Biomol Spectrosc. 2021 Jan 15;245:118892. doi: 10.1016/j.saa.2020.118892. Epub 2020 Aug 28. PMID: 32916423.
- Li L, Li L, Wang C, Liu K. Synthesis of nitrogen-doped and amino acid-functionalized graphene quantum dots from glycine, and their application to the fluorometric determination of ferric ion. Microchimica Acta. 2015; 182: 763-770.
- Xue G, Yu S, Qiang Z, Xiuying L, Tang Lijun, Jiangrong L. Application of maleimide modified graphene quantum dots and porphyrin fluorescence resonance energy transfer in the design of ''turn-on'' fluorescence sensors for biothiols. Anal Chim Acta. 2020 Apr 29;1108:46-53. doi: 10.1016/j.aca.2020.01.062. Epub 2020 Jan 29. PMID: 32222243.
- Yu D, Zhang X, Qi Y, Ding S, Cao S, Zhu A, Shi G. Pb2+-modified graphene quantum dots as a fluorescent probe for biological aminothiols mediated by an inner filter effect. Sensors and Actuators B: Chemical. 2016; 235: 394-400.
- Zhu Q, Mao H, Li J, Hua J, Wang J, Yang R, Li Z. A glycine-functionalized graphene quantum dots synthesized by a facile post-modification strategy for a sensitive and selective fluorescence sensor of mercury ions. Spectrochim Acta A Mol Biomol Spectrosc. 2021 Feb 15;247:119090. doi: 10.1016/j.saa.2020.119090. Epub 2020 Oct 17. PMID: 33137626.
- Zhang P , Zhao X , Ji Y , Ouyang Z , Wen X , Li J , Su Z , Wei G . Electrospinning graphene quantum dots into a nanofibrous membrane for dual-purpose fluorescent and electrochemical biosensors. J Mater Chem B. 2015 Mar 28;3(12):2487-2496. doi: 10.1039/c4tb02092h. Epub 2015 Feb 18. PMID: 32262123.
- Safaei-Ghomi J, Elyasi Z, Babaei P. N-doped graphene quantum dots modified with CuO (0D)/ZnO (1D) heterojunction as a new nanocatalyst for environmental being one-pot synthesis of monospiro derivatives. New Journal of Chemistry. 2021; 45(3): 1269-1277.
- Xu T, Wang D, Dong L, Shen H, Lu W, Chen W. Graphitic carbon nitride comodified by zinc phthalocyanine and graphene quantum dots for the efficient photocatalytic degradation of refractory contaminants. Applied Catalysis B: Environmental. 2019; 244: 96-106.
- Yu Z, Ma W, Wu T, Wen J, Zhang Y, Wang L, He Y, Chu H, Hu M. Coumarin-Modified Graphene Quantum Dots as a Sensing Platform for Multicomponent Detection and Its Applications in Fruits and Living Cells. ACS Omega. 2020 Mar 25;5(13):7369-7378. doi: 10.1021/acsomega.9b04387. PMID: 32280878; PMCID: PMC7144171.
- Yang S, Zhu C, Sun J, He P, Yuan N, Ding J, Ding G. Triphenylphosphine modified graphene quantum dots: spectral modulation for full spectrum of visible light with high quantum yield. RSC Advances. 2015; 5(42): 33347-33350.
- Guo R, Zhou S, Li Y, Li X, Fan L, Voelcker NH. Rhodamine-Functionalized Graphene Quantum Dots for Detection of Fe(3+) in Cancer Stem Cells. ACS Appl Mater Interfaces. 2015 Nov 4;7(43):23958-66. doi: 10.1021/acsami.5b06523. Epub 2015 Oct 26. PMID: 26317667.
- Song H, Wang Y, Wang J, Wang G, He J, Wei H, Luo S. Preparation and biodistribution of 131I-labeled graphene quantum dots. Journal of Radioanalytical and Nuclear Chemistry. 2018; 316:685-690.
- Gharepapagh E, Fakhari A, Firuzyar T, Shomali A, Azimi F. Preparation, biodistribution and dosimetry study of Tc-99m labeled N-doped graphene quantum dot nanoparticles as a multimodular radiolabeling agent. New Journal of Chemistry. 2021; 45(8): 3909-3919.
- Chong Y, Ma Y, Shen H, Tu X, Zhou X, Xu J, Dai J, Fan S, Zhang Z. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials. 2014 Jun;35(19):5041-8. doi: 10.1016/j.biomaterials.2014.03.021. Epub 2014 Mar 28. PMID: 24685264.
- Kim DJ, Yoo JM, Suh Y, Kim D, Kang I, Moon J, Park M, Kim J, Kang KS, Hong BH. Graphene Quantum Dots from Carbonized Coffee Bean Wastes for Biomedical Applications. Nanomaterials (Basel). 2021 May 28;11(6):1423. doi: 10.3390/nano11061423. PMID: 34071339; PMCID: PMC8228242.
- Tang H, Li Y, Kakinen A, Andrikopoulos N, Sun Y, Kwak E, Davis TP, Ding F, Ke PC. Graphene quantum dots obstruct the membrane axis of Alzheimer's amyloid beta. PCCP. 2022; 24(1): 86-97.
- Rostamzadeh F, Shadkam-Farrokhi M, Jafarinejad-Farsangi S, Najafipour H, Ansari-Asl Z, Yeganeh-Hajahmadi M. PEGylated Graphene Quantum Dot Improved Cardiac Function in Rats with Myocardial Infarction: Morphological, Oxidative Stress, and Toxicological Evidences. Oxid Med Cell Longev. 2021 Nov 20;2021:8569225. doi: 10.1155/2021/8569225. PMID: 34845418; PMCID: PMC8627339.
- Abdullah-Al-Nahain, Lee JE, In I, Lee H, Lee KD, Jeong JH, Park SY. Target delivery and cell imaging using hyaluronic acid-functionalized graphene quantum dots. Mol Pharm. 2013 Oct 7;10(10):3736-44. doi: 10.1021/mp400219u. Epub 2013 Sep 19. PMID: 24007260.
- Jia Q, Li Z, Guo C, Huang X, Song Y, Zhou N, Wang M, Zhang Z, He L, Du M. A γ-cyclodextrin-based metal-organic framework embedded with graphene quantum dots and modified with PEGMA via SI-ATRP for anticancer drug delivery and therapy. Nanoscale. 2019 Nov 21;11(43):20956-20967. doi: 10.1039/c9nr06195a. Epub 2019 Oct 29. PMID: 31660562.
- Wei Z, Yin X, Cai Y, Xu W, Song C, Wang Y, Zhang J, Kang A, Wang Z, Han W. Antitumor effect of a Pt-loaded nanocomposite based on graphene quantum dots combats hypoxia-induced chemoresistance of oral squamous cell carcinoma. Int J Nanomedicine. 2018 Mar 13;13:1505-1524. doi: 10.2147/IJN.S156984. PMID: 29559779; PMCID: PMC5856292.
- Nafiujjaman M, Revuri V, Park HK, Kwon IK, Cho KJ, Lee YK. Enhanced Photodynamic Properties of Graphene Quantum dot Conjugated Ce6 Nanoparticles for Targeted Cancer Therapy and Imaging. Chemistry Letters. 2016; 45(8): 997-999.
- Wang C, Chen Y, Xu Z, Chen B, Zhang Y, Yi X, Li J. Fabrication and characterization of novel cRGD modified graphene quantum dots for chemophotothermal combination therapy. Sens. Actuators B: Chem. 2020; 309: 127732.
- Cao Y, Dong H, Yang Z, Zhong X, Chen Y, Dai W, Zhang X. Aptamer-Conjugated Graphene Quantum Dots/Porphyrin Derivative Theranostic Agent for Intracellular Cancer-Related MicroRNA Detection and Fluorescence-Guided Photothermal/Photodynamic Synergetic Therapy. ACS Appl Mater Interfaces. 2017 Jan 11;9(1):159-166. doi: 10.1021/acsami.6b13150. Epub 2016 Dec 22. PMID: 27957830.
- Yang S, Wang X, He P, Xu A, Wang G, Duan J, Shi Y, Ding G. Graphene Quantum Dots with Pyrrole N and Pyridine N: Superior Reactive Oxygen Species Generation Efficiency for Metal-Free Sonodynamic Tumor Therapy. Small. 2021 Mar;17(10):e2004867. doi: 10.1002/smll.202004867. Epub 2021 Jan 28. PMID: 33511794.
- Yan H, Wang Q, Wang J, Shang W, Xiong Z, Zhao L, Sun X, Tian J, Kang F, Yun SH. Planted Graphene Quantum Dots for Targeted, Enhanced Tumor Imaging and Long-Term Visualization of Local Pharmacokinetics. Adv Mater. 2023 Apr;35(15):e2210809. doi: 10.1002/adma.202210809. Epub 2023 Mar 3. PMID: 36740642; PMCID: PMC10374285.
- Qi L, Pan T, Ou L, Ye Z, Yu C, Bao B, Wu Z, Cao D, Dai L. Biocompatible nucleus-targeted graphene quantum dots for selective killing of cancer cells via DNA damage. Commun Biol. 2021 Feb 16;4(1):214. doi: 10.1038/s42003-021-01713-1. PMID: 33594275; PMCID: PMC7886873.
- Kuo WS, Shao YT, Huang KS, Chou TM, Yang CH. Antimicrobial Amino-Functionalized Nitrogen-Doped Graphene Quantum Dots for Eliminating Multidrug-Resistant Species in Dual-Modality Photodynamic Therapy and Bioimaging under Two-Photon Excitation. ACS Appl Mater Interfaces. 2018 May 2;10(17):14438-14446. doi: 10.1021/acsami.8b01429. Epub 2018 Apr 19. PMID: 29620851.
- Zubair M, Husain FM, Fatima F, Oves M, Ansari MA, Almari M, Dai L. (Eds.). Graphene quantum dots biomedical and environmental sustainability applications. Woodhead Publishing. 2023; 83-100.
- Kortel M, Mansuriya BD, Vargas Santana N, Altintas Z. Graphene Quantum Dots as Flourishing Nanomaterials for Bio-Imaging, Therapy Development, and Micro-Supercapacitors. Micromachines (Basel). 2020 Sep 18;11(9):866. doi: 10.3390/mi11090866. PMID: 32962061; PMCID: PMC7570118.
- Wang C, Wu C, Zhou X, Han T, Xin X, Wu J, Zhang J, Guo S. Enhancing cell nucleus accumulation and DNA cleavage activity of anti-cancer drug via graphene quantum dots. Sci Rep. 2013 Oct 4;3:2852. doi: 10.1038/srep02852. PMID: 24092333; PMCID: PMC3790198.
- González-Gaitán M, Stenmark H. Endocytosis and signaling: a relationship under development. Cell. 2003 Nov 26;115(5):513-21. doi: 10.1016/s0092-8674(03)00932-2. PMID: 14651844.
- Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018 May;19(5):313-326. doi: 10.1038/nrm.2017.132. Epub 2018 Feb 7. PMID: 29410531.
- Hillaireau H, Couvreur P. Nanocarriers' entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009 Sep;66(17):2873-96. doi: 10.1007/s00018-009-0053-z. Epub 2009 Jun 5. PMID: 19499185.
- Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci. 2003 Dec 1;116(Pt 23):4707-14. doi: 10.1242/jcs.00840. PMID: 14600257.
- Sun YP, Zhou B, Lin Y, Wang W, Fernando KA, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PG, Yang H, Kose ME, Chen B, Veca LM, Xie SY. Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc. 2006 Jun 21;128(24):7756-7. doi: 10.1021/ja062677d. PMID: 16771487.
- Gonçalves H, Esteves da Silva JC. Fluorescent carbon dots capped with PEG200 and mercaptosuccinic acid. J Fluoresc. 2010 Sep;20(5):1023-8. doi: 10.1007/s10895-010-0652-y. Epub 2010 Mar 30. PMID: 20352303.
- Chandra A, Deshpande S, Shinde DB, Pillai VK, Singh N. Mitigating the Cytotoxicity of Graphene Quantum Dots and Enhancing Their Applications in Bioimaging and Drug Delivery. ACS Macro Lett. 2014 Oct 21;3(10):1064-1068. doi: 10.1021/mz500479k. Epub 2014 Oct 6. PMID: 35610793.
- Chong Y, Ma Y, Shen H, Tu X, Zhou X, Xu J, Dai J, Fan S, Zhang Z. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials. 2014 Jun;35(19):5041-8. doi: 10.1016/j.biomaterials.2014.03.021. Epub 2014 Mar 28. PMID: 24685264.
- Sachdev A, Matai I, Gopinath P. Implications of surface passivation on physicochemical and bioimaging properties of carbon dots. RSC Advances. 2014; 4(62): 20915–20921.
- Gao T, Wang X, Yang LY, He H, Ba XX, Zhao J, Jiang FL, Liu Y. Red, Yellow, and Blue Luminescence by Graphene Quantum Dots: Syntheses, Mechanism, and Cellular Imaging. ACS Appl Mater Interfaces. 2017 Jul 26;9(29):24846-24856. doi: 10.1021/acsami.7b05569. Epub 2017 Jul 12. PMID: 28675929.
- Havrdová M, Hola K, Skopalik J, Tomankova K, Petr M, Cepe K, Polakova K, Tucek J, Bourlinos AB, Zboril R. Toxicity of carbon dots–Effect of surface functionalization on the cell viability, reactive oxygen species generation and cell cycle. Carbon. 2016; 99: 238-248.
- Ngafwan N, Rasyid H, Abood ES, Abdelbasset WK, Al-Shawi SG, Bokov D, Jalil AT Study on novel fluorescent carbon nanomaterials in food analysis. Food Science and Technology. 2021; 42(1).
- Xu H, Zhou S, Xiao L, Wang H, Li S, Yuan Q. Fabrication of a nitrogen-doped graphene quantum dot from MOF-derived porous carbon and its application for highly selective fluorescence detection of Fe 3+. Journal of Materials Chemistry C. 2015; 3(1): 291–297.
- Yang JM, Hu XW, Liu YX, Zhang W. Fabrication of a carbon quantum dots-immobilized zirconium-based metal-organic framework composite fluorescence sensor for highly sensitive detection of 4-nitrophenol. Microporous and Mesoporous Materials. 2019; 274: 149-154.
- Wei X, Wang Y, Huang Y, Fan C. Composite ZIF-8 with CQDs for boosting visible-light-driven photocatalytic removal of NO. Journal of Alloys and Compounds. 2019; 802: 467-476.
- Wang XM, Geng ZM, Sun H, Cong HL, Yu B. Preparation of C-ZIF-8 composite nanoparticles. Integrated Ferroelectrics. 2018; 188(1): 130-135.
- Camlik G, Ozakca I, Bilakaya B, Ozcelikay AT, Velaro AJ, Wasnik S, Degim IT. Development of composite carbon quantum dots-insulin formulation for oral administration. Journal of Drug Delivery Science and Technology. 2022; 76: 103833.
- Su M, Liu H, Ge L, Wang Y, Ge S, Yu J, Yan M. Aptamer-Based electrochemiluminescent detection of MCF-7 cancer cells based on carbon quantum dots coated mesoporous silica nanoparticles. Electrochimica Acta. 2014; 146: 262-269.
- Nozaki T , Kakuda T , Pottathara YB , Kawasaki H . A nanocomposite of N-doped carbon dots with gold nanoparticles for visible light active photosensitisers. Photochem Photobiol Sci. 2019 May 15;18(5):1235-1241. doi: 10.1039/c9pp00035f. PMID: 30843904.
- Ghosh Chaudhuri R, Paria S. Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev. 2012 Apr 11;112(4):2373-433. doi: 10.1021/cr100449n. Epub 2011 Dec 28. PMID: 22204603.
- Alijani HQ, Fathi A, Amin HIM, Nobre MAL, Akbarizadeh MR, Khatami M, Jalil AT, Naderifar M, Dehkordi FS, Shafiee A. Biosynthesis of core–shell α-Fe2O3@ Au nanotruffles and their biomedical applications. Biomass Conversion and Biorefinery. 2022; 1-15.
- Eskalen H, Urus S, C ̈omertpay S, Kurt AH, Ozgan S. Microwave-assisted ultrafast synthesis of carbon quantum dots from linter: Fluorescence cancer imaging and human cell growth inhibition properties. Industrial Crops and Products. 2020; 147: 112209.
- Kong RM, Yang A, Wang Q, Wang Y, Ma L, Qu F. Uricase based fluorometric determination of uric acid based on the use of graphene quantum dot@ silver core-shell nanocomposites. Microchemical Journal. 2018; 185-186: 1-8.
- Pei H, Zhu S, Yang M, Kong R, Zheng Y, Qu F. Graphene oxide quantum dots@silver core-shell nanocrystals as turn-on fluorescent nanoprobe for ultrasensitive detection of prostate specific antigen. Biosens Bioelectron. 2015 Dec 15;74:909-14. doi: 10.1016/j.bios.2015.07.056. Epub 2015 Jul 29. PMID: 26257182.
- Liu Q, Guo B, Rao Z, Zhang B, Gong JR. Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging. Nano Lett. 2013 Jun 12;13(6):2436-41. doi: 10.1021/nl400368v. Epub 2013 May 15. PMID: 23675758.
- Zhang J, Yu SH. Carbon dots: large-scale synthesis, sensing and bioimaging. Materials Today. 2016; 19(6): 382-393.
- Wang B, Waterhouse GI, Qu X, Yang B, Lu S. Carbon Dots in Bioimaging, Biosensing and Therapeutics: A Comprehensive Review. Small Science. 2022; 2200012.
- Li LP, Ren XF, Bai PR, Liu Y, Xu WY, Xie J. Near-infrared emission carbon dots for bio-imaging applications. New Carbon Materials. 2021; 36(6): 632-638.
- Du J, Xu N, Fan J, Sun W, Peng X. Carbon Dots for in vivo Bioimaging and Theranostics. Small. 2019 Aug;15(32):e1805087. doi: 10.1002/smll.201805087. Epub 2019 Feb 18. PMID: 30779301.
- Zhou N, Hao Z, Zhao X, Maharjan S, Zhu S, Song Y, Yang B, Lu L. A novel fluorescent retrograde neural tracer: cholera toxin B conjugated carbon dots. Nanoscale. 2015 Oct 14;7(38):15635-42. doi: 10.1039/c5nr04361a. Epub 2015 Aug 18. PMID: 26285001.
- Yuan F, Ding L, Li Y, Li X, Fan L, Zhou S, Fang D, Yang S. Multicolor fluorescent graphene quantum dots colorimetrically responsive to all-pH and a wide temperature range. Nanoscale. 2015 Jul 21;7(27):11727-33. doi: 10.1039/c5nr02007g. Epub 2015 Jun 23. PMID: 26102292.
- Fan Z, Li Y, Li X, Fan L, Zhou S, Fang D, Yang S. Surrounding media sensitive photoluminescence of boron-doped graphene quantum dots for highly fluorescent dyed crystals, chemical sensing and bioimaging. Carbon. 2014; 70: 149-156.
- Wang N, Zheng AQ, Liu X, Chen JJ, Yang T, Chen ML, Wang JH. Deep Eutectic Solvent-Assisted Preparation of Nitrogen/Chloride-Doped Carbon Dots for Intracellular Biological Sensing and Live Cell Imaging. ACS Appl Mater Interfaces. 2018 Mar 7;10(9):7901-7909. doi: 10.1021/acsami.8b00947. Epub 2018 Feb 21. PMID: 29424521.
- Sadhanala HK, Nanda KK. Boron-doped carbon nanoparticles: Sizeindependent color tunability from red to blue and bioimaging applications. Carbon. 2016; 96: 166-173.
- Ruan S, Qian J, Shen S, Chen J, Zhu J, Jiang X, He Q, Yang W, Gao H. Fluorescent carbonaceous nanodots for noninvasive glioma imaging after angiopep-2 decoration. Bioconjug Chem. 2014 Dec 17;25(12):2252-9. doi: 10.1021/bc500474p. Epub 2014 Nov 11. PMID: 25387274.
- Li H, Yan X, Qiao S, Lu G, Su X. Yellow-Emissive Carbon Dot-Based Optical Sensing Platforms: Cell Imaging and Analytical Applications for Biocatalytic Reactions. ACS Appl Mater Interfaces. 2018 Mar 7;10(9):7737-7744. doi: 10.1021/acsami.7b17619. Epub 2018 Feb 22. PMID: 29441784.
- Xue M , Zhao J , Zhan Z , Zhao S , Lan C , Ye F , Liang H . Dual functionalized natural biomass carbon dots from lychee exocarp for cancer cell targetable near-infrared fluorescence imaging and photodynamic therapy. Nanoscale. 2018 Oct 4;10(38):18124-18130. doi: 10.1039/c8nr05017a. PMID: 30255925.
- Sharma V , Tiwari P , Mobin SM . Sustainable carbon-dots: recent advances in green carbon dots for sensing and bioimaging. J Mater Chem B. 2017 Dec 7;5(45):8904-8924. doi: 10.1039/c7tb02484c. Epub 2017 Nov 6. PMID: 32264117.
- Jana P, Dev A. Carbon Quantum Dots: A Promising Nanocarrier for Bioimaging and Drug Delivery in Cancer. Materials Today Communications. 2022; 25: 104068.
- Azizi M, Valizadeh H, Shahgolzari M, Talebi M, Baybordi E, Dadpour MR, Salehi R, Mehrmohammadi M. Synthesis of Self-Targeted Carbon Dot with Ultrahigh Quantum Yield for Detection and Therapy of Cancer. ACS Omega. 2020 Sep 17;5(38):24628-24638. doi: 10.1021/acsomega.0c03215. PMID: 33015480; PMCID: PMC7528278.
- Majood M, Garg P, Chaurasia R, Agarwal A, Mohanty S, Mukherjee M. Carbon Quantum Dots for Stem Cell Imaging and Deciding the Fate of Stem Cell Differentiation. ACS Omega. 2022 Aug 11;7(33):28685-28693. doi: 10.1021/acsomega.2c03285. PMID: 36033677; PMCID: PMC9404166.
- Hamd-Ghadareh S, Salimi A, Fathi F, Bahrami S. An amplified comparative fluorescence resonance energy transfer immunosensing of CA125 tumor marker and ovarian cancer cells using green and economic carbon dots for bio-applications in labeling, imaging and sensing. Biosens Bioelectron. 2017 Oct 15;96:308-316. doi: 10.1016/j.bios.2017.05.003. Epub 2017 May 4. PMID: 28525848.
- Demir B, Lemberger MM, Panagiotopoulou M, Medina Rangel PX, Timur S, Tse Sum Bui B, Wegener J, Haupt K. Tracking hyaluronan: molecularly targeted fluorescence imaging guided by carbon dots. Biomaterials. 2022; 250: 120056.
- Wegner KD, Hildebrandt N. Quantum dots: bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem Soc Rev. 2015 Jul 21;44(14):4792-834. doi: 10.1039/c4cs00532e. PMID: 25777768.
- Liu T, Liu Z, Wu J, Zhang K, An H, Hu Z, Deng S, Li X. Broadband near-infrared persistent luminescence in Ni 2+-doped transparent glass-ceramic ZnGa 2 O 4. New Journal of Chemistry. 2022; 46(3): 851-856.
- Li Y, Dong H, Tao Q, Ye C, Yu M, Li J, Zhou H, Yang S, Ding G, Xie X. Enhancing the magnetic relaxivity of MRI contrast agents via the localized superacid microenvironment of graphene quantum dots. Biomaterials. 2020 Aug;250:120056. doi: 10.1016/j.biomaterials.2020.120056. Epub 2020 Apr 17. PMID: 32339859.
- Yang ST, Cao L, Luo PG, Lu F, Wang X, Wang H, Meziani MJ, Liu Y, Qi G, Sun YP. Carbon dots for optical imaging in vivo. J Am Chem Soc. 2009 Aug 19;131(32):11308-9. doi: 10.1021/ja904843x. PMID: 19722643; PMCID: PMC2739123.
- Tao H, Yang K, Ma Z, Wan J, Zhang Y, Kang Z, Liu Z. in vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small. 2012 Jan 23;8(2):281-90. doi: 10.1002/smll.201101706. Epub 2011 Nov 18. PMID: 22095931.
- Gao G, Jiang YW, Yang J, Wu FG. Mitochondria-targetable carbon quantum dots for differentiating cancerous cells from normal cells. Nanoscale. 2017 Nov 30;9(46):18368-18378. doi: 10.1039/c7nr06764j. PMID: 29143843.
- Huang X, Zhang F, Zhu L, Choi K, Guo N, Guo J, Tackett K, Anilkumar P, Liu G, Quan Q, Sun YP. Graphene-Based Materials for Cell Imaging and Drug Delivery. Nano Letters. 2013; 13(4): 5684–5693.
- Wang Y, Meng Y, Wang S, Li C, Shi W, Chen J, Wang J, Huang R. Direct Solvent-Derived Polymer-Coated Nitrogen-Doped Carbon Nanodots with High Water Solubility for Targeted Fluorescence Imaging of Glioma. Small. 2015 Aug 5;11(29):3575-81. doi: 10.1002/smll.201403718. Epub 2015 Mar 24. PMID: 25808813.
- Licciardello N, Hunoldt S, Bergmann R, Singh G, Mamat C, Faramus A, Ddungu JL, Pieters RJ, Otto C, Zimmermann M, Hildebrandt N. Actively targeted silica nanoparticles enable specific single-cell imaging in living zebrafish. ACS Nano. 2019; 13(4): 4953–4968.
- Li X, Wang Y, Chen Y, Zhou P, Wei K, Wang H, Wang J, Fang H, Zhang S. Hierarchically constructed selenium-doped bone-mimetic nanoparticles promote ROS-mediated autophagy and apoptosis for bone tumor inhibition. Biomaterials. 2020 Oct;257:120253. doi: 10.1016/j.biomaterials.2020.120253. Epub 2020 Jul 23. PMID: 32738660.
- Maashi MS, Al-Mualm M, Al-Awsi GRL, Opulencia MJC, Al-Gazally ME, Abdullaev B, Abdelbasset WK, Ansari MJ, Jalil AT, Alsaikhan F, Shalaby MN, Mustafa YF. Apigenin alleviates resistance to doxorubicin in breast cancer cells by acting on the JAK/STAT signaling pathway. Mol Biol Rep. 2022 Sep;49(9):8777-8784. doi: 10.1007/s11033-022-07727-0. Epub 2022 Jul 8. PMID: 35804214.
- Mintz KJ, Cilingir EK, Nagaro G, Paudyal S, Zhou Y, Khadka D, Huang S, Graham RM, Leblanc RM. Development of Red-Emissive Carbon Dots for Bioimaging through a Building Block Approach: Fundamental and Applied Studies. Bioconjug Chem. 2022 Jan 19;33(1):226-237. doi: 10.1021/acs.bioconjchem.1c00544. Epub 2021 Dec 16. PMID: 34914353.
- Su R, Yan H, Jiang X, Zhang Y, Li P, Su W. Orange-red to NIR emissive carbon dots for antimicrobial, bioimaging and bacteria diagnosis. Journal of Materials Chemistry B. 2022; 10(5): 1250-1264.
- Chu X, Zhang P, Wang Y, Sun B, Liu Y, Zhang Q, Feng W, Li Z, Li K, Zhou N. Near-infrared carbon dot-based platform for bioimaging and photothermal/photodynamic/quaternary ammonium triple synergistic sterilization triggered by single NIR light source. Carbon. 2021; 176: 126-138.
- Naji GH, Fareed NY, Al-Zheery WH. Dendrimer as a recent drug delivery system: An overview. Journal of Biomedi Biochem. 2022; 1(3): 57-69.
- Feng T, Chua HJ, Zhao Y. Reduction-Responsive Carbon Dots for Real-Time Ratiometric Monitoring of Anticancer Prodrug Activation in Living Cells. ACS Biomater Sci Eng. 2017 Aug 14;3(8):1535-1541. doi: 10.1021/acsbiomaterials.7b00264. Epub 2017 Jun 13. PMID: 33429640.
- Gong X, Zhang Q, Gao Y, Shuang S, Choi MM, Dong C. Phosphorus and Nitrogen Dual-Doped Hollow Carbon Dot as a Nanocarrier for Doxorubicin Delivery and Biological Imaging. ACS Appl Mater Interfaces. 2016 May 11;8(18):11288-97. doi: 10.1021/acsami.6b01577. Epub 2016 Apr 26. PMID: 27088972.
- Zhang M, Wang W, Zhou N, Yuan P, Su Y, Shao M, Chi C, Pan F. Near-infrared light triggered photo-therapy, in combination with chemotherapy using magnetofluorescent carbon quantum dots for effective cancer treating. Carbon. 2017; 118: 752-764.
- Gao N, Yang W, Nie H, Gong Y, Jing J, Gao L, Zhang X. Turn-on theranostic fluorescent nanoprobe by electrostatic self-assembly of carbon dots with doxorubicin for targeted cancer cell imaging, in vivo hyaluronidase analysis, and targeted drug delivery. Biosens Bioelectron. 2017 Oct 15;96:300-307. doi: 10.1016/j.bios.2017.05.019. Epub 2017 May 10. PMID: 28511113.
- Ashrafizadeh M, Mohammadinejad R, Kailasa SK, Ahmadi Z, Afshar EG, Pardakhty A. Carbon dots as versatile nanoarchitectures for the treatment of neurological disorders and their theranostic applications: A review. Adv Colloid Interface Sci. 2020 Apr;278:102123. doi: 10.1016/j.cis.2020.102123. Epub 2020 Feb 19. PMID: 32087367.
- Wu H, Su W, Xu H, Zhang Y, Li Y, Li X, Fan L. Applications of carbon dots on tumour theranostics. View. 2021; 2: 20200061.
- Shi X, Meng H, Sun Y, Qu L, Lin Y, Li Z, Du D. Far-Red to Near-Infrared Carbon Dots: Preparation and Applications in Biotechnology. Small. 2019 Nov;15(48):e1901507. doi: 10.1002/smll.201901507. Epub 2019 Jun 6. PMID: 31168960.
- Jia Q, Ge J, Liu W, Zheng X, Chen S, Wen Y, Zhang H, Wang P. A Magnetofluorescent Carbon Dot Assembly as an Acidic H2 O2 -Driven Oxygenerator to Regulate Tumor Hypoxia for Simultaneous Bimodal Imaging and Enhanced Photodynamic Therapy. Adv Mater. 2018 Mar;30(13):e1706090. doi: 10.1002/adma.201706090. Epub 2018 Feb 13. PMID: 29436031.
- Ding H, Yu SB, Wei JS, Xiong HM. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano. 2016 Jan 26;10(1):484-91. doi: 10.1021/acsnano.5b05406. Epub 2015 Dec 8. PMID: 26646584.
- Yang W, Zhang H, Lai J, Peng X, Hu Y, Gu W, Ye L. Carbon dots with redshifted photoluminescence by fluorine doping for optical bio-imaging. Carbon. 2018; 128: 78-85.
- Wang H, Mukherjee S, Yi J, Banerjee P, Chen Q, Zhou S. Biocompatible Chitosan-Carbon Dot Hybrid Nanogels for NIR-Imaging-Guided Synergistic Photothermal-Chemo Therapy. ACS Appl Mater Interfaces. 2017 Jun 7;9(22):18639-18649. doi: 10.1021/acsami.7b06062. Epub 2017 May 22. PMID: 28485151.
- Guo XL, Ding ZY, Deng SM, Wen CC, Shen XC, Jiang BP, Liang H. A novel strategy of transition-metal doping to engineer absorption of carbon dots for near-infrared photothermal/photodynamic therapies. Carbon. 2018; 134: 519-530.
- Li J, Regev G, Patel SK, Patton D, Sweeney Y, Graebing P, Grab S, Wang L, Sant V, Rohan LC. Rational Design of a Multipurpose Bioadhesive Vaginal Film for Co-Delivery of Dapivirine and Levonorgestrel. Pharmaceutics. 2019 Dec 18;12(1):1. doi: 10.3390/pharmaceutics12010001. PMID: 31861267; PMCID: PMC7023193.
- Tyo KM, Lasnik AB, Zhang L, Mahmoud M, Jenson AB, Fuqua JL, Palmer KE, Steinbach-Rankins JM. Sustained-release Griffithsin nanoparticle-fiber composites against HIV-1 and HSV-2 infections. J Control Release. 2020 May 10;321:84-99. doi: 10.1016/j.jconrel.2020.02.006. Epub 2020 Feb 5. PMID: 32035194; PMCID: PMC7170769.
- Bluhmki T, Bitzer S, Gindele JA, Schruf E, Kiechle T, Webster M, Schymeinsky J, Ries R, Gantner F, Bischoff D, Garnett J, Heilker R. Development of a miniaturized 96-Transwell air-liquid interface human small airway epithelial model. Sci Rep. 2020 Aug 3;10(1):13022. doi: 10.1038/s41598-020-69948-2. PMID: 32747751; PMCID: PMC7400554.
- Cao X, Coyle JP, Xiong R, Wang Y, Heflich RH, Ren B, Gwinn WM, Hayden P, Rojanasakul L. Invited review: human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives. In Vitro Cell Dev Biol Anim. 2021 Feb;57(2):104-132. doi: 10.1007/s11626-020-00517-7. Epub 2020 Nov 11. PMID: 33175307; PMCID: PMC7657088.
- Karami MH, Pourmadadi M, Abdouss M, Kalaee MR, Moradi O, Rahdar A, Díez-Pascual AM. Novel chitosan/γ-alumina/carbon quantum dot hydrogel nanocarrier for targeted drug delivery. Int J Biol Macromol. 2023 Aug 15;251:126280. doi: 10.1016/j.ijbiomac.2023.126280. Epub ahead of print. PMID: 37591420.
- Yaqub N, Wayne G, Birchall M, Song W. Recent advances in human respiratory epithelium models for drug discovery. Biotechnol Adv. 2022 Jan-Feb;54:107832. doi: 10.1016/j.biotechadv.2021.107832. Epub 2021 Sep 2. PMID: 34481894.
- Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine. 2015 Sep;75(1):68-78. doi: 10.1016/j.cyto.2015.05.014. Epub 2015 Jun 9. PMID: 26070934; PMCID: PMC4532591.
- Whitsett JA. Airway Epithelial Differentiation and Mucociliary Clearance. Ann Am Thorac Soc. 2018 Nov;15(Suppl 3):S143-S148. doi: 10.1513/AnnalsATS.201802-128AW. PMID: 30431340; PMCID: PMC6322033.