More Information
Submitted: September 15, 2023 | Approved: October 17, 2023 | Published: October 18, 2023
How to cite this article: Nogueira GC, da Silva LC, Hatanaka DM, Iasi M, Zacharias RSB, et al. Management of Congenital Cervical Teratoma with Application of EXIT Protocol - Case Report. Clin J Obstet Gynecol. 2023; 6: 172-178.
DOI: 10.29328/journal.cjog.1001147
Copyright License: © 2023 Nogueira GC, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Congenital cervical immature teratoma; Immature teratoma; Cervical teratoma; Extrauterine intrapartum treatment procedure; Case report
Abbreviations: EXIT: Extrauterine Intrapartum Treatment Procedure; MRI: Magnetic Resonance Imaging; SIMV: Synchronized Intermittent Mandatory Ventilation; FiO2: Fraction of Inspired Oxygen; PIP: Peak of Inspiratory Pressure; PEEP: Positive End-Expiratory Pressure; RR: Respiratory Rate; SatO2: Saturation of Oxygen
Management of Congenital Cervical Teratoma with Application of EXIT Protocol - Case Report
Gleydson Cavalcante Nogueira<1*, Larissa Cassemiro da Silva2, Diná Mie Hatanaka3, Marcelo Iasi1, Romy Schmidt Brock Zacharias1 and Mariano Tamura Vieira Gomes1
1Hospital Israelita Albert Einstein, Brazil
2Pontifical Catholic University of Campinas, Brazil
3Hospital Moriah, Brazil
*Address for Correspondence: Gleydson Cavalcante Nogueira, Hospital Israelita Albert Einstein, Brazil, Email: [email protected]
Background: Congenital teratomas are relatively rare neoplasms, which occurs in about 1:20,000 to 1:80,000 births, and only 1.5% to 5% of which are neoplasm of the cervical. They can be diagnosed through ultrasound during pregnancy and, if not properly handled, have a high mortality rate. Airway compression is a secondary complication following mortality.
Case report: A solid-cystic mass was identified in the anterior cervical region of a 30-week-old fetus during an ultrasound scan. EXIT (Ex-Utero Intrapartum Treatment)-to-airway procedure was performed by a multidisciplinary team composed of obstetricians, anesthesiologists, neonatologists and pediatric surgeons to remove the neoplasm. The procedure occurred upon delivery of the fetus, resulting in a positive outcome with neonatal survival. In this case, the fetus was in breech position, and, differently from the usual EXIT protocol, it had to be completely extracted before guaranteeing airway flow.
Conclusion: Although congenital teratomas are a rare condition with complex treatment, it is possible to achieve a satisfactory outcome when adequate planning and protocol are established.
Fetal teratomas are rare, the reported incidence is between 0.07 and 2.8 in 1000 pregnancies [1]. The most common location of teratoma is the sacrococcygeal region (70% - 80%), followed by the head and neck, chest, and retroperitoneum. It is reported that teratomas in fetal head or neck account for less than 5%. The pathophysiology of teratoma remains unknown [2]. The incidence is higher among females with female to male ratio being 3:1 [3]. No relation to the maternal age or specific ethnicity could be established. Speculations regarding probable genetic changes on comparative genomic hybridizations are being sought [4].
Diagnosis is usually made in the second or third trimester of pregnancy by ultrasound, where they appear as masses of mixed echogenicity with cystic and solid parts, calcifications and blood perfusion of varying degree. Typically, they are not associated with abnormal genetic findings [1]. Prenatal imaging with close attention to the rate of growth of the mass, the location of the teratoma and its mass effects on airway and major neck vessels, should be an integral part of the initial and ongoing evaluation [5].
Fetal cervical and oropharyngeal tumors’ major problems may be related to the invasion of facial, pulmonary, or central nervous system structures, with the consequent development of polyhydramnios and fetal hydrops. Also, newborns may present an airway obstruction and/or injury due to an intrinsic lesion in the larynx or trachea, or related to an external compression that oropharyngeal or neck masses may produce [6]. Cervical teratomas usually occur in the third trimester and are not always detected prenatally. It is therefore essential to assess the fetal neck when polyhydramnios is noted at any third trimester scan, to maximize detection and ensure appropriate multidisciplinary healthcare can be organized to optimize postnatal survival [7].
Perinatal management of large neck masses is always challenging because of difficulty in accessing the distorted airway and if unidentified has a mortality of 80% - 100% [8]. Cervical teratomas are usually treated with prompt complete surgical excision, which is effective and associated with good long-term outcomes. Procedures such as ex-utero intrapartum treatment (EXIT) are used for lesions that have been diagnosed during the prenatal period to prevent airway obstruction at birth and to increase the chances of postnatal survival [9]. The main, but not sole, indication for an Ex-utero Intrapartum Treatment (EXIT) delivery is an airway obstruction due to either laryngeal atresia or tumors in the head and neck region. It is used to secure the fetal airway while maintaining uteroplacental gas exchange and fetal hemodynamic stability through the umbilical circulation [10]. In the following case, because the fetus was in breech position, multidisciplinary team planning was required to utilize a modified EXIT procedure, ensuring a secure airway to avoid a potentially fatal outcome.
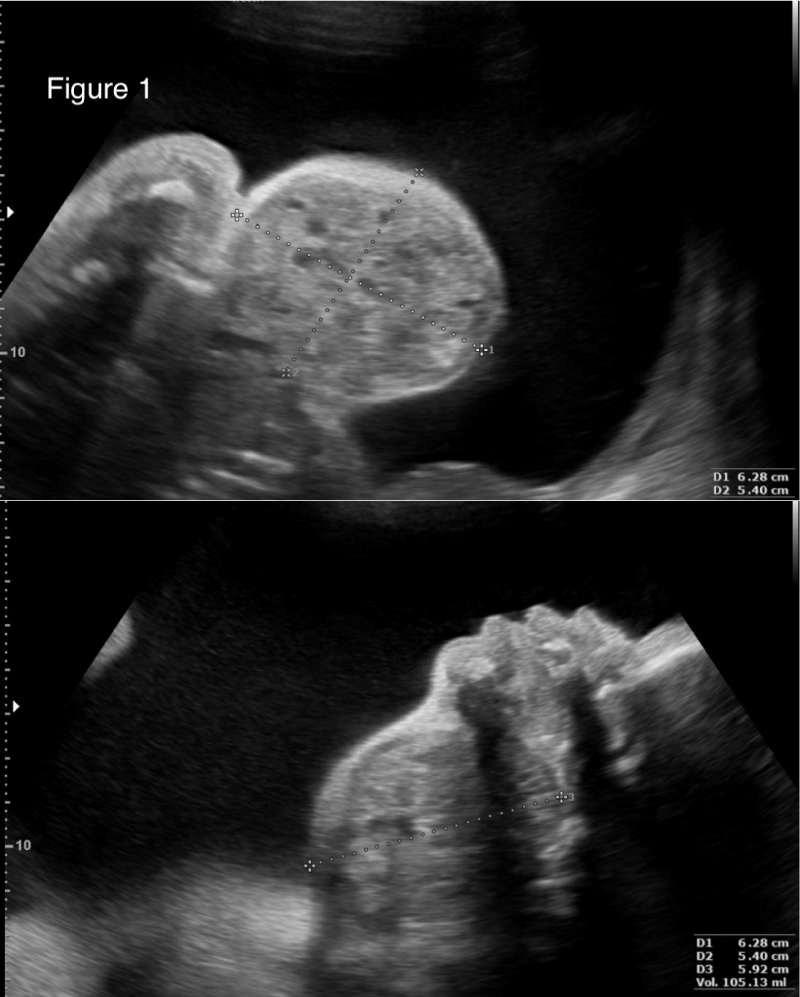
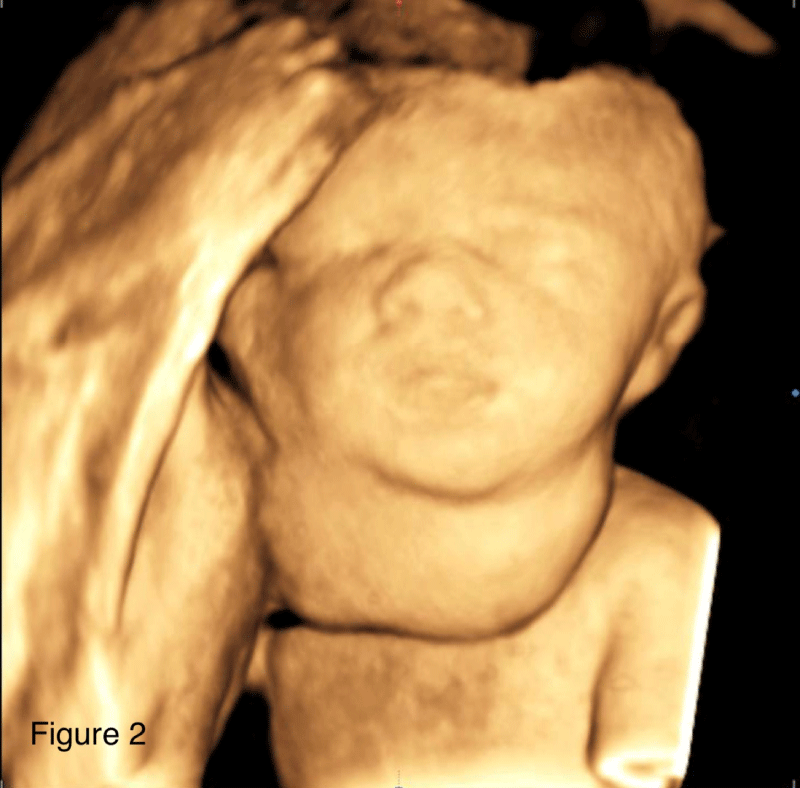
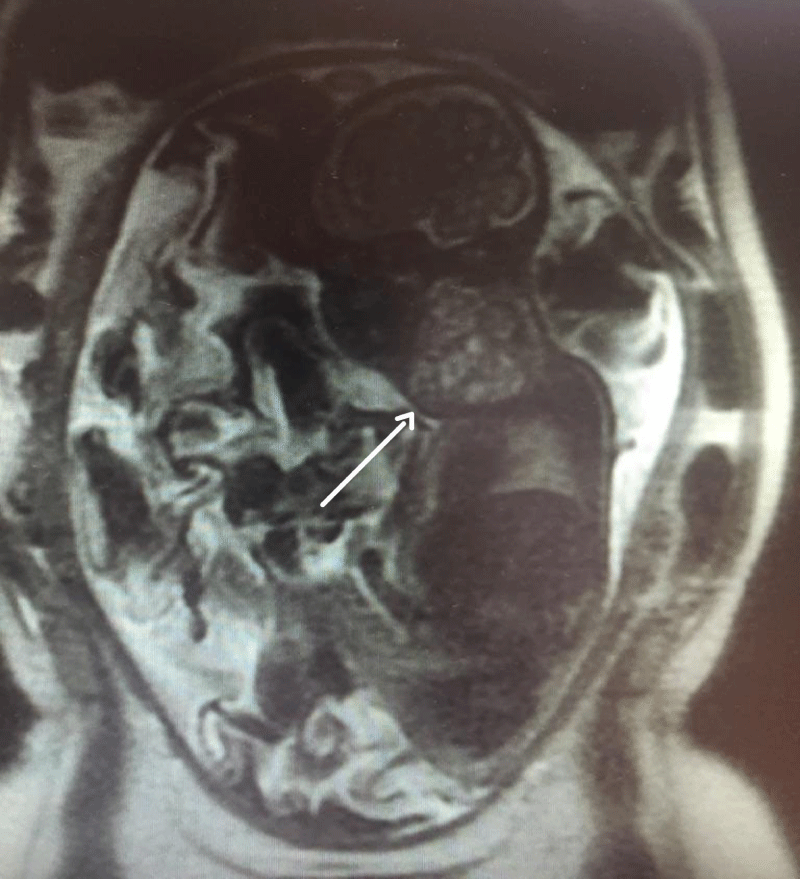
Patient is a 42-year-old gravida 3, white, married, with bachelor's degree, without comorbidities, previous surgeries or relevant family medical history, with two previous natural births; one being a healthy full-term baby and one stillbirth at 26 weeks for unknown reason. The patient’s current pregnancy, with a female fetus, presented no alterations during the prenatal follow-up morphology scans of the 1st and 2nd trimesters. At 27 weeks, gestational diabetes was diagnosed, and her glycemic range controlled through a healthy diet. At 31 weeks and 2 days (11/06/2017), the patient went into preterm labor, presenting lower back pain and abdominal distension, but good fetal movement; cervical dilation was 2 cm and the fundus height was 34 cm. Atosiban was prescribed to inhibit labor and tests were requested to assess fetal well-being. At 31 weeks and 3 days cardiotocography (CTG) monitoring showed a reassuring pattern, and the obstetric ultrasound evidenced polyhydramnios as the only alteration, with AFI of 36.52 cm. A second Doppler ultrasonography was performed the following day at 31 weeks and 4 days, which showed that the 1502 g weighing fetus presented a 6,3 x 5,4 x 5,9 cm (104 cm3) tumor formation in the anterior cervical region with a solid-cystic appearance, different echogenicity, regular contours, and low vascularity, which was compressing cervical structures (Figures 1,2). A diagnostic test was executed through fetal magnetic resonance imaging (MRI) and the patient was admitted at a high-risk obstetric referral center to further analyze the case. The MRI showed a large, heterogeneous, well delimited, solid cystic formation located in the bilateral anterior cervical region, with a larger component to the right, measuring about 6.8 x 5.4 x 4.7 cm (90 cm3), determining lateral displacement of the oropharynx to the left, progressive reduction of the airway caliber, and more pronounced in the laryngeal-tracheal transition (Figure 3). The hypothesis of a congenital cervical teratoma or cervical lymphangioma was raised, leading to a multidisciplinary team approach, composed of obstetricians, anesthesiologists, neonatal intensivists and pediatric surgeons. The patient was treated with corticosteroid therapy followed by a programmed c-section and EXIT procedure at 32 weeks and 1 day (gestational age), to reduce the risk of spontaneous preterm birth. The patient, with continuous vital signs monitorization, successfully received an epidural anesthesia with lidocaine at L4-L5 region. Surgical procedure was initiated with the completion of a suprapubic transverse incision, using the Pfannenstiel incision technique. Optimized anesthetic technique was conducted, associating general anesthesia with 437.5 mg of propofol, 300 mcg of fentanyl, 476.25 mcg of remifentanil and 100 mg of rocuronium. The patient's intubation procedure was accomplished using a no. 7 cannula. Following, the cesarean section was initialized through a low-segment transverse uterine incision, and after the opening of the uterus there was a large amount of clear liquid output. The EXIT procedure usually recommends the extraction of the cephalic pole only, until the child’s airway flow can be guaranteed by intubation, or in some cases tracheostomy, but in this case fetal extraction had to be performed by the pelvic pole because the baby was in breech position, followed by careful release of the cephalic pole. With an unclamped umbilical cord, ensuring fetus-placental circulation, the newborn was successfully intubated under direct laryngoscopy, using a no. 2.5, uncuffed endotracheal tube. Because the infant’s airway shifted to the left, it was difficult to pass the tube and the use of a guidewire was necessary to fulfill the procedure. A pediatric surgeon was in the OR in case a tracheostomy or an emergency tumor surgical removal was necessary. The umbilical cord was only clamped seven minutes after birth, with the confirmation of a steady airflow measured through capnography. Next, placenta was removed followed by uterine suture, hemostasis and standardized steps to successfully finish the surgery, with a total length of one hour. The estimated blood loss was 1475 ml. There was no other maternal complication than symptomatic anemia (hemoglobin 8,7 g/dl), for which she received intravenous iron and was discharged with a hemoglobin of 13,6 g/dl.
Figure 1: Obstetric ultrasound evidencing a 6,3 x 5,4 x 5,9 cm (105 cm3) tumor formation in the anterior cervical region with a solid-cystic appearance, different echogenicity, regular contours, and low vascularity.
Figure 2: 3D ultrasound imaging of the fetus cervical mass.
Figure 3: Magnetic Resonance Imaging evidencing a large heterogeneous, well-delimited solid-cystic expansive formation, located in the bilateral anterior cervical region, with a larger component on the right, measuring about 6.8 x 5.4 x 4.7 cm.
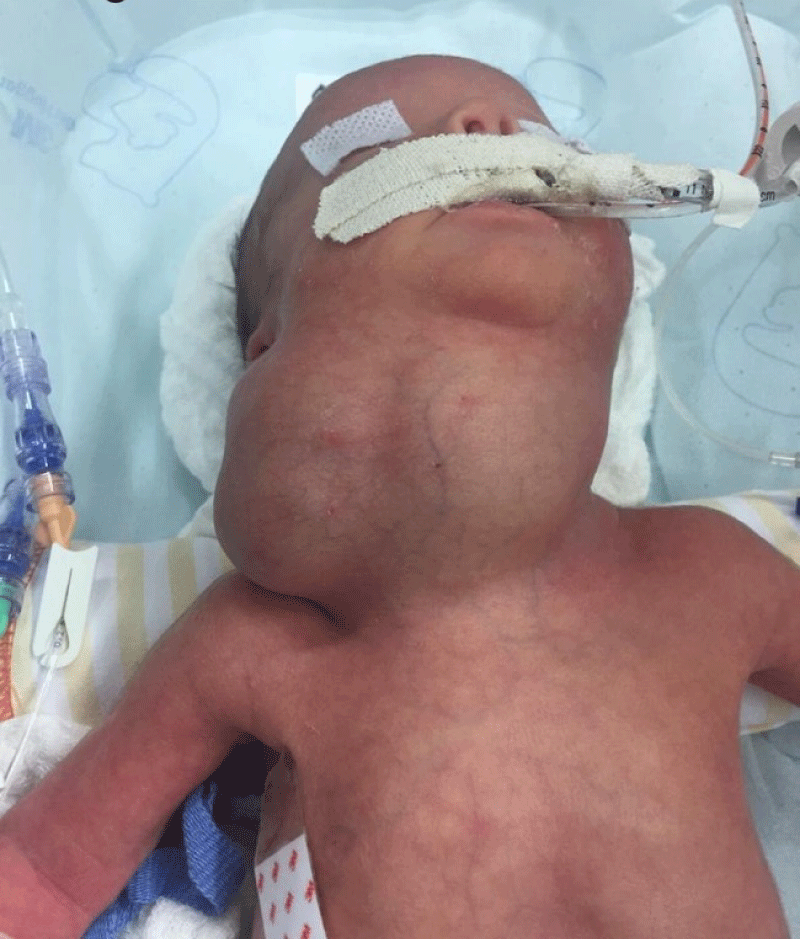
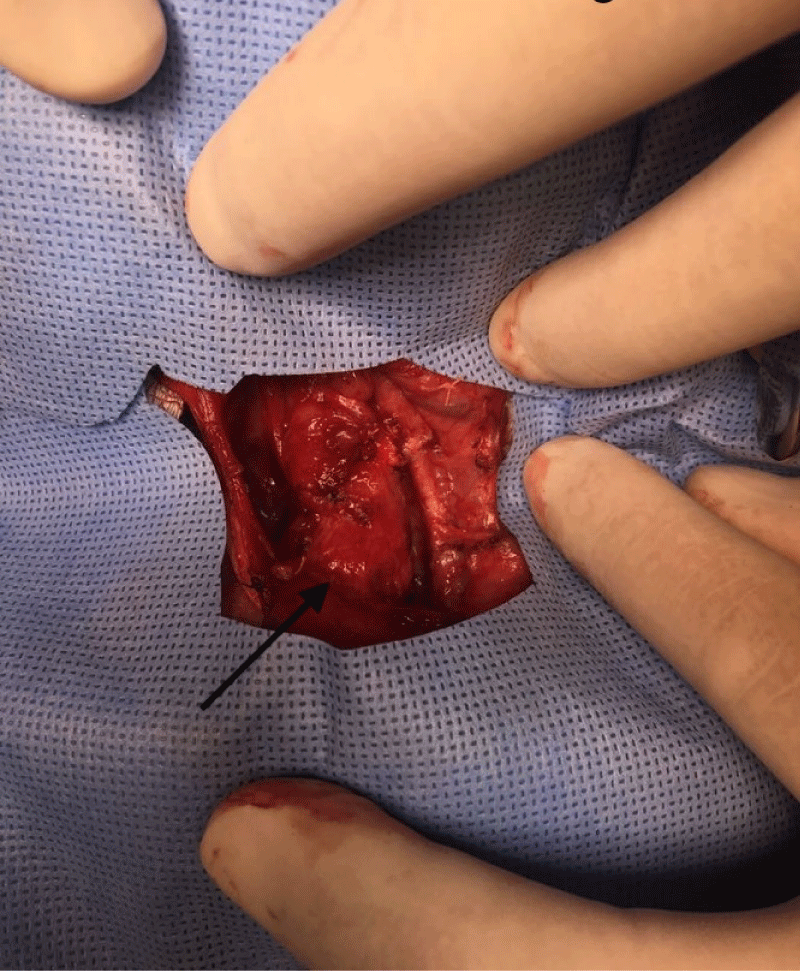
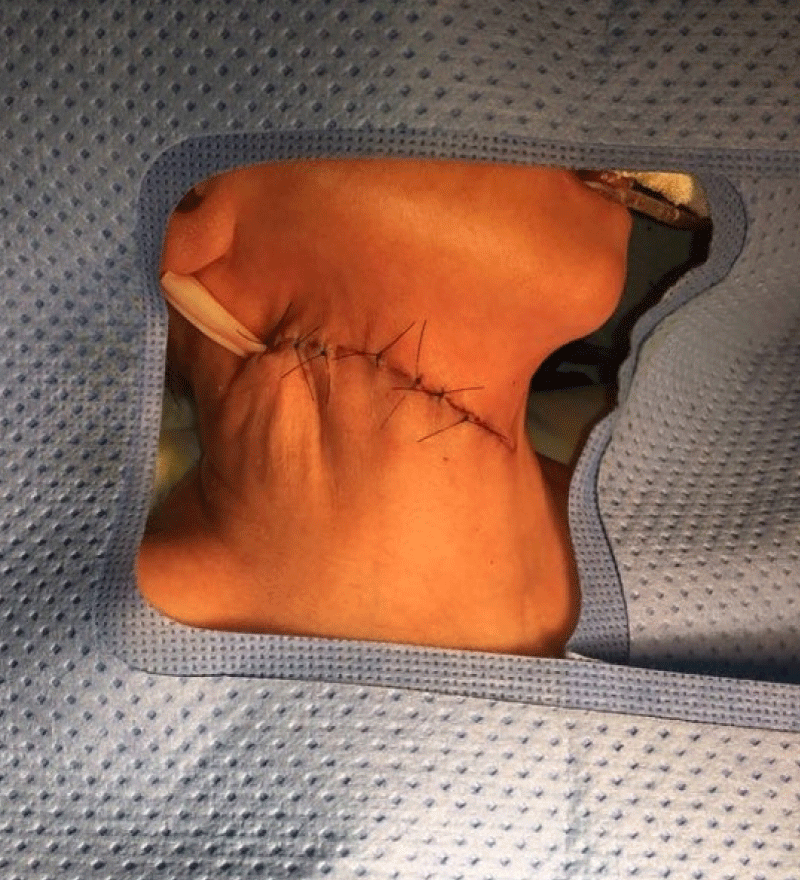
Meanwhile, the newborn, whose birth weight was 1,905 g, height 41 cm and APGAR 7/8 (neonate under maternal anesthesia), was under the care of the neonatology team. The infant underwent a nasogastric decompression procedure with the use of a NG tube no. 8, which showed little gastric content (Figure 4). No resuscitation maneuvers were required. The newborn was placed on mechanical ventilation (Synchronized Intermittent Mandatory Ventilation - SIMV with a Fraction of Inspired Oxygen - FiO2 of 35%, a Peak of Inspiratory Pressure – PIP of 13, a Positive End-expiratory Pressure - PEEP of 7, a Respiratory Rate – RR of 30 and a Saturation of Oxygen – SatO2: 94%) during the investigation period through neonatal surgical resolution and a single dose of 100 mg/kg surfactant was handled with good response of the ventilatory-derived parameters. The procedure for removal of the neoplasm was performed by a pediatric surgery team 6 days after birth and the cervical mass was removed without complications or glandular, vascular or nervous lesions (Figures 5, 6).
Figure 4: Newborn after orotracheal intubation with use of n˚. 2.5 tube and passage of nasogastric tube n° 8.
Figure 5: Meticulous dissection of the cervical area for tumor mass excision.
Figure 6: Cervical region aspect in the immediate postoperative period.
The tumor was sent for pathological analysis, which identified neoplasia composed of cells originated from three embryonic leaflets. Over 50% of the content was composed of immature neural component, heterogeneous foci of mature cartilaginous tissue, skeletal muscle and bone. Cystic areas formed by glandular epithelium and mature squamous, and mature thyroid tissue in the periphery, measuring 6.3 x 5.2 x 3.1 cm and weighing 72 g, concluding as a grade 3 immature teratoma.
The infant was discharged after 45 days of hospitalization with no complications. She is currently a healthy 4-year-old, with no signs of recurrence, sequelae or further treatment needed.
Congenital neck masses are developmental malformations that present with a wide spectrum of clinical symptoms and signs [11]. Congenital teratoma is one of these rare forms of neoplasms, occurring in approximately 0.07 to 2.8 in 1000 pregnancies [1].
The pathophysiology of teratoma remains unknown. Neither maternal age nor specific ethnicities were found to be associated with this tumor [12]. Infants with these tumors can be born with fatal respiratory distress due to mass effect or related pulmonary hypoplasia, especially if not identified during the prenatal period [13]. Neck teratomas have a propensity towards airway obstruction. In addition, newborns can have intrinsic lesion in the larynx or trachea. Cervical teratoma can be highly vascularized, which can lead to lethal blood loss [14]. Elevated intrathoracic pressure due to the mass effect may decrease venous return, causing cardiac failure, fetal and/or placental hydrops [15].
Histologically analyzing, teratomas originate from pluripotent germ cells and are composed of derivatives of at least one of the three germ layers (ectoderm, mesoderm and endoderm), with the sacral region being the most common (40% - 45%) [2]. Therefore, their histological profile is heterogenous and includes cystic or solid areas along with mature or immature components [16].
Antenatal counseling helps the parents to better understand the natural history, fetal intervention, and perinatal management of these tumors, which differ dramatically depending on their size and location [17]. While teratomas are among the most seen antenatally diagnosed tumors, masses located in the face and neck region represent a minority of cases, estimated at 10% - 15% [18].
Prenatal diagnosis can be achieved using 2D ultrasound. Both ultrasound and magnetic resonance imaging (MRI) enable the differentiation between solid or partly solid tumors such as fetal goiter, teratoma or neuroblastoma and cystic or multicystic lesions like lymphangioma and hemangioma [19]. Ultrasound can be useful in determining the size and consistency of the lesions, and often shows a heterogeneous mass at the anterolateral portion of the fetal neck, with hyperextension of the neck, associated with calcifications, as well as solid and cystic components [20,21] . Another important aspect of antenatal management of fetal neck tumors is an assessment of amniotic fluid volume. The increase of Amniotic Fluid Index (AFI) and development of polyhydramnios indicate on impaired fetal swallowing because of esophagus and trachea displacement and compression [19].
MRI typically provides the best imaging delineation. Teratomas tend to be heterogeneous on both T1 and T2 weighted sequences depending upon the composition of tissue types constituting the lesion. Cervical teratomas often have a close relationship with the thyroid gland. Absence of the ipsilateral thyroid gland or splaying of the thyroid tissue around the mass is further supportive of a teratoma diagnosis [20]. In a study conducted by Matos, et al. MRI demonstrated the relationship between cervical masses and adjacent organs and allowed 3D virtual reconstruction of the airways. There was complete agreement between the prenatal diagnosis of cervical masses on MRI and postnatal diagnosis [22]. The largest vertical pocket measurement and mass effect on the trachea were the most contributory MRI parameters that predicted significant morbidity in fetuses with masses of the face and neck, along with other significant parameters [23].
Initially, like other fetal tumors, neck masses may negatively affect the fetal cardiac function because of increased cardiac afterload and preload. Secondly, they may affect the patency of fetal airways, including trachea, which may have fatal consequences after birth. In addition, big neck tumors, especially teratomas, may cause fetal lung hypoplasia, because of wedging the lungs into the apices of chest cavity [19]. Teratoma should be evaluated as part of the differential diagnosis of neck masses, and confirmed or excluded before deciding on definitive therapy, despite its rarity [24].
Since head and neck anatomy is topographically complex, and the region is densely populated by vital nerves as well as vascular and lymphatic structures, perinatal management is a complex and challenging procedure. Therefore, an expert multidisciplinary team is the key to success, and it requires an understanding of the types of lesions and knowledge of the prenatal images that would best delineate the anatomic defect [25].
EXIT is a technique first described in 1990 by Zerella and Finberg and it is designed to allow partial fetal delivery via cesarean section while a safe fetal airway is established [26]. It can provide time to manage the fetal airway while maintaining placental blood circulation to the fetus [27]. After identifying patients in need of EXIT, patient management begins with the creation of a multidisciplinary team that includes obstetricians, radiologists, obstetric anesthesiologists, pediatric surgeons, anesthesiologists, and neonatologists [28].
Upon delivery of the fetal head, neck, and one or both upper limbs, a stepwise, sequential attempt to secure the neonates airway is performed. The placenta remains in utero to maintain its circulation and subsequently the continuance of fetal oxygenation [26]. The surgical incision can be either midline or lower-transverse (Pfannenstiel incision) or, following hysterotomy, lower-transverse (Keer incision) where the fetus is partially delivered (head, upper chest, and at least one upper limb). As it is necessary to avoid work of breathing and body movements to achieve orotracheal intubation, it may be necessary the use of fetal anesthesia immediately after pulling out an upper limb, intramuscularly in the deltoid [29]. Once the newborn’s airway is secured, the umbilical cord is clamped and delivery of the infant completed [26]. Fetal interventions performed during EXIT can include endotracheal intubation, tracheostomy, mass excision, removal of a temporary tracheal occlusive device, ECMO cannulation, and others [30].
Although EXIT was initially designed to reverse tracheal occlusion performed on fetuses with a severe congenital diaphragmatic hernia, its indications have expanded over the years [31]. Direct tracheal involvement (compression or obstruction), tracheal deviation via mass effect, and anterior mass location documented on antenatal imaging were associated with an increased need for invasive airway maneuvers at birth. Poly-hydramnios, an indicator of aerodigestive obstruction, has been shown to indicate a complicated airway in the context of congenital cervical mass [32].
Initially the mother will be the primary focus and her designated anesthetist will most likely administer a general anesthetic with rapid sequence induction [30]. Successful anesthetic management of the EXIT is possible with both general anesthesia and regional anesthesia. However, there is a significant preponderance towards general anesthesia [34]. It allows for the use of inhalation agents which cause tocolysis, described by Braden, et al. as complete uterine relaxation [33]. Tocolysis is the cornerstone of fetal surgery. Surgical manipulations or uterine incisions may initiate uterine contractions. These contractions can compress the umbilical vessels, compromise the placental blood flow, and even lead to placental abruption from the endometrium, leading to complete disruption of fetal blood flow, necessitating emergency fetal delivery [35].
Although no maternal deaths during EXIT procedures have been reported, excessive postpartum hemorrhage can necessitate maternal blood transfusion in up to 13% of cases and postoperative wound complication may be 10-times greater than standard cesarean delivery. Reported risks for children born by EXIT include increased rates of traumatic facial nerve paralysis, feeding difficulties, obstructive sleep apnea, and speech development delay, although it is impossible to discern if this is due to the procedure or merely associated with the pathology for which EXIT is required [36].
Rodriguez, et al. reported that neck masses had a mortality rate of up to 40% prior to EXIT, and those that did survive had a high morbidity rate [37]. Byun, et al. believed that EXIT procedure can reduce the mortality rate of neonates with neck masses to as low as 8% [38]. Risks for neonatal demise at EXIT (or soon after) include fetal teratoma, low birth weight, and prematurity [39]. Patients with antepartum fetal conditions requiring delivery by EXIT are better managed in fetal care centers with adequate expertise due to complexity of the antenatal, intrapartum, and postpartum management of these cases [40].
The presented case is challenging due to breech presentation and preterm labor, which demanded urgent and precise care for a modified EXIT procedure, that was successfully applied by a multidisciplinary team, resulting in a good outcome for both mother and the infant.
Despite being a rare condition, with adequate prenatal follow-up, a prompt diagnose is possible to be achieved. Diagnose during pregnancy can be attained through ultrasound scans and is a decisive factor for the outcome. Surgery performed by a multidisciplinary team combined with the EXIT protocol and intrapartum airway assurance are essential strategies for neonatal survival.
- Simonini C, Strizek B, Berg C, Gembruch U, Mueller A, Heydweiller A, Geipel A. Fetal teratomas - A retrospective observational single-center study. Prenat Diagn. 2021 Feb;41(3):301-307. doi: 10.1002/pd.5872. Epub 2020 Dec 17. PMID: 33242216.
- Li D, Gao H, Zheng W, Jin C, Huang Y, Pan S. Case report: Fetal cervical immature teratoma and copy number variations. Front Oncol. 2022 Aug 15;12:843268. doi: 10.3389/fonc.2022.843268. PMID: 36046039; PMCID: PMC9423720.
- Oommen J, Mohammed H, Ayyappan Kutty S, Mammen A, Kalathingal K, Vellani Thamunni C, Sivadasan A, Nair S. Neonatal Teratoma: Craniofacial Treatment. J Craniofac Surg. 2019 Jan;30(1):e17-e19. doi: 10.1097/SCS.0000000000004906. PMID: 30480623.
- Mohanty MK, Sahu P, Jaiswal AA, Singal R, Gupta S, Kohli G, Garg AK. A huge immature cervical teratoma; antenatal diagnosis, and its management - an unusual entity. J Clin Neonatol. 2013 Jan;2(1):42-5. doi: 10.4103/2249-4847.109249. PMID: 24027746; PMCID: PMC3761953.
- Shamshirsaz AA, Aalipour S, Stewart KA, Nassr AA, Furtun BY, Erfani H, Sundgren NC, Cortes MS, Donepudi RV, Lee TC, Mehta DK, Kravitz ES, Asl NM, Espinoza J, Belfort MA. Perinatal characteristics and early childhood follow up after ex-utero intrapartum treatment for head and neck teratomas by prenatal diagnosis. Prenat Diagn. 2021 Mar;41(4):497-504. doi: 10.1002/pd.5894. Epub 2021 Feb 9. PMID: 33386645.
- García-Díaz L, Chimenea A, de Agustín JC, Pavón A, Antiñolo G. Ex-Utero Intrapartum Treatment (EXIT): indications and outcome in fetal cervical and oropharyngeal masses. BMC Pregnancy Childbirth. 2020 Oct 7;20(1):598. doi: 10.1186/s12884-020-03304-0. PMID: 33028259; PMCID: PMC7541246.
- Holloway S. Antenatal detection of a thyrocervical teratoma in the third trimester - A case report. Ultrasound. 2022 Nov;30(4):328-332. doi: 10.1177/1742271X221091728. Epub 2022 May 9. PMID: 36969530; PMCID: PMC10034648.
- Jain P, Prasad A, Rahul KM, Ankur K. Difficult Airway of Fetus: Making a Safe Ex Utero Intrapartum Treatment. J Indian Assoc Pediatr Surg. 2021 Nov-Dec;26(6):448-450. doi: 10.4103/jiaps.JIAPS_226_20. Epub 2021 Nov 12. PMID: 34912147; PMCID: PMC8637992.
- Alharbi ST, Alsaadi AS, Yosuph AU, Abdulhameed FD, Arkoubi MM. Diagnostic imaging and surgical management of a congenital cervical teratoma. J Taibah Univ Med Sci. 2017 Jul 4;13(1):83-86. doi: 10.1016/j.jtumed.2017.05.013. PMID: 31435307; PMCID: PMC6694921.
- Mesas Burgos C, Tiblad E, Papatziamos G, Jörnvall H, Conner P. EXIT – säkraste förlossningsmetod för foster med livshotande luftvägshinder - Samlade erfarenheter från Karolinska universitetssjukhuset [EXIT: Experiences from Karolinska University Hopsital]. Lakartidningen. 2019 May 15;116:FHWM. Swedish. PMID: 31192393.
- Pupić-Bakrač J, Pupić-Bakrač A, Novaković J, Skitarelić N. Congenital Neck Masses. J Craniofac Surg. 2021 Jun 1;32(4):1417-1420. doi: 10.1097/SCS.0000000000007122. PMID: 33170821.
- Hochwald O, Gil Z, Gordin A, Winer Z, Avrahami R, Abargel E, Khoury A, Lehavi A, Abecassis P, Eldor L, Ben-Izhak O, Borenstein-Levin L, Stienberg R, Kugelman A. Three-step management of a newborn with a giant, highly vascularized, cervical teratoma: a case report. J Med Case Rep. 2019 Mar 10;13(1):73. doi: 10.1186/s13256-019-1976-0. PMID: 30851737; PMCID: PMC6409158.
- Patel S, Kunnath AJ, Gallant JN, Belcher RH. Surgical Management and Outcomes of Pediatric Congenital Head and Neck Teratomas: A Scoping Review. OTO Open. 2023 Aug 9;7(3):e66. doi: 10.1002/oto2.66. PMID: 37565058; PMCID: PMC10410334.
- Stomnaroska O, Kocovski G, Zdravkovski P, Ilievski B, Jovanovic R, Petrusevska G. Large Neck Teratoma in a Newborn with Respiratory Distress Syndrome. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2021 Apr 23;42(1):105-108. doi: 10.2478/prilozi-2021-0009. PMID: 33894120.
- Beckers K, Faes J, Deprest J, Delaere PR, Hens G, De Catte L, Naulaers G, Claus F, Hermans R, Vander Poorten VLM. Long-term outcome of pre- and perinatal management of congenital head and neck tumors and malformations. Int J Pediatr Otorhinolaryngol. 2019 Jun;121:164-172. doi: 10.1016/j.ijporl.2019.03.018. Epub 2019 Mar 16. PMID: 30917301.
- Malhotra S, Negi P, Sagar P. A Case of Cervical Teratoma in an Infant. Indian J Otolaryngol Head Neck Surg. 2022 Dec;74(Suppl 3):6519-6523. doi: 10.1007/s12070-021-02942-w. Epub 2021 Oct 20. PMID: 36742920; PMCID: PMC9895199.
- Peiró JL, Sbragia L, Scorletti F, Lim FY, Shaaban A. Management of fetal teratomas. Pediatr Surg Int. 2016 Jul;32(7):635-47. doi: 10.1007/s00383-016-3892-3. Epub 2016 Apr 25. PMID: 27112491.
- Rubio EI. Imaging of the fetal oral cavity, airway and neck. Pediatr Radiol. 2021 Jun;51(7):1122-1133. doi: 10.1007/s00247-020-04851-6. Epub 2021 May 12. PMID: 33978788.
- Kornacki J, Skrzypczak J. Fetal neck tumors - antenatal and intrapartum management. Ginekol Pol. 2017;88(5):266-269. doi: 10.5603/GP.a2017.0050. PMID: 28580573.
- Wolter NE, Siegele B, Cunningham MJ. Cystic cervical teratoma: A diagnostic and management challenge. Int J Pediatr Otorhinolaryngol. 2017 Apr;95:97-100. doi: 10.1016/j.ijporl.2017.02.016. Epub 2017 Feb 14. PMID: 28576544.
- Cansaran S, Cerrah Celayir A, Moralıoğlu S, Ayvacı H, Tuğrul S, Ovalı F, Çetiner H. The EXIT for Prenatally Diagnosed Cervical Cystic Teratoma: A Case Report. J Neonatal Surg. 2015 Apr 1;4(2):18. PMID: 26034712; PMCID: PMC4447471.
- Paula Pinho Matos A, Teixeira Castro P, de Barros Duarte L, Dutra Moraes Barbosa A, Daltro P, Fazecas T, Nogueira R, Werner H, Araujo Júnior E. Prenatal diagnosis of cervical masses by magnetic resonance imaging and 3D virtual models: perinatal and long-term follow-up outcomes. J Matern Fetal Neonatal Med. 2020 Jul;33(13):2181-2189. doi: 10.1080/14767058.2018.1543393. Epub 2018 Nov 20. PMID: 30458651.
- Ng TW, Xi Y, Schindel D, Beavers A, Santiago-Munoz P, Bailey AA, Twickler DM. Fetal Head and Neck Masses: MRI Prediction of Significant Morbidity. AJR Am J Roentgenol. 2019 Jan;212(1):215-221. doi: 10.2214/AJR.18.19753. Epub 2018 Nov 13. PMID: 30422714.
- Ali IM, Yaşar M, Abdi AA, Dırken ES. Successful surgıcal management of a giant neck teratoma in a newborn Baby: A case report. Ann Med Surg (Lond). 2022 Sep 15;82:104694. doi: 10.1016/j.amsu.2022.104694. PMID: 36268334; PMCID: PMC9577653.
- Zheng W, Gai S, Qin J, Qiu F, Li B, Zou Y. Role of prenatal imaging in the diagnosis and management of fetal facio-cervical masses. Sci Rep. 2021 Jan 14;11(1):1385. doi: 10.1038/s41598-021-80976-4. PMID: 33446872; PMCID: PMC7809128.
- Manchali MM, Sharabu C, Latha M, Kumar L. A rare case of oropharyngeal teratoma diagnosed antenatally with MRI. J Clin Imaging Sci. 2014 Mar 21;4:15. doi: 10.4103/2156-7514.129261. PMID: 24744972; PMCID: PMC3988610.
- Masahata K, Soh H, Tachibana K, Sasahara J, Hirose M, Yamanishi T, Ibuka S, Okuyama H, Usui N. Clinical outcomes of ex utero intrapartum treatment for fetal airway obstruction. Pediatr Surg Int. 2019 Aug;35(8):835-843. doi: 10.1007/s00383-019-04494-1. Epub 2019 Jun 4. PMID: 31165248.
- Chung W, Lim C. Intraoperative management for ex-utero intrapartum treatment: focusing on the fetus. Anesth Pain Med (Seoul). 2021 Oct;16(4):329-337. doi: 10.17085/apm.21097. Epub 2021 Oct 29. PMID: 35139613; PMCID: PMC8828620.
- Orrego G J, Mosquera-Hernández JC, Ardila-Giraldo S, Torres-Canchala L, Alzate E, Benavidez JP. Técnica EXIT como manejo de la vía aérea en masas gigantes congénitas de cuello [Ex-Utero Intrapartum Treatment for airway management in congenital giant neck masses]. Rev Chil Pediatr. 2020 Jun;91(3):398-404. Spanish. doi: 10.32641/rchped.vi91i3.1385. PMID: 32730521.
- Bence CM, Wagner AJ. Ex utero intrapartum treatment (EXIT) procedures. Semin Pediatr Surg. 2019 Aug;28(4):150820. doi: 10.1053/j.sempedsurg.2019.07.003. Epub 2019 Jul 22. PMID: 31451172.
- García-Díaz L, Chimenea A, de Agustín JC, Pavón A, Antiñolo G. Ex-Utero Intrapartum Treatment (EXIT): indications and outcome in fetal cervical and oropharyngeal masses. BMC Pregnancy Childbirth. 2020 Oct 7;20(1):598. doi: 10.1186/s12884-020-03304-0. PMID: 33028259; PMCID: PMC7541246.
- Barrette LX, Morales CZ, Oliver ER, Gebb JS, Feygin T, Lioy J, Howell LJ, Hedrick HL, Jackson OA, Adzick NS, Javia LR. Risk factor analysis and outcomes of airway management in antenatally diagnosed cervical masses. Int J Pediatr Otorhinolaryngol. 2021 Oct;149:110851. doi: 10.1016/j.ijporl.2021.110851. Epub 2021 Jul 21. PMID: 34311168.
- Silva M. Ex utero intrapartum treatment (EXIT) procedure: Indications and procedural considerations. Journal of Perioperative Nursing. 2019 Dec 1. doi: https://doi.org/10.26550/2209-1092.1068.
- Kumar K, Miron C, Singh SI. Maternal anesthesia for EXIT procedure: A systematic review of literature. J Anaesthesiol Clin Pharmacol. 2019 Jan-Mar;35(1):19-24. doi: 10.4103/joacp.JOACP_302_17. PMID: 31057234; PMCID: PMC6495613.
- Joshi H, Packiasabapathy S. Anesthetic Management of Ex Utero Intrapartum Treatment (EXIT) and Fetal Surgery. 2023 May 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 34283463.
- Cash H, Bly R, Masco V, Dighe M, Cheng E, Delaney S, Ma K, Perkins JA. Prenatal Imaging Findings Predict Obstructive Fetal Airways Requiring EXIT. Laryngoscope. 2021 Apr;131(4):E1357-E1362. doi: 10.1002/lary.28959. Epub 2020 Aug 8. PMID: 32770766.
- Rodríguez MJ, Moreno-Cid M, Pascual A, Rubio A, López M, Moñux A, García J, Cabra J, Saavedra P. Delivery strategy for foetuses with cervical mass: The EXIT procedure. J Obstet Gynaecol. 2016;36(1):64-5. doi: 10.3109/01443615.2015.1030603. Epub 2015 Jul 28. PMID: 26219996.
- Byun SH, Lee SY, Hong SY, Ryu T, Kim BJ, Jung JY. Use of the GlideScope video laryngoscope for intubation during ex utero intrapartum treatment in a fetus with a giant cyst of the 4th branchial cleft: A case report. Medicine (Baltimore). 2016 Sep;95(39):e4931. doi: 10.1097/MD.0000000000004931. PMID: 27684833; PMCID: PMC5265926.
- Porter H, Trivedi A, Marquez M, Gibson P, Melov SJ, Mishra U, Jani P, Cheng AT, Nayyar R, Alahakoon TI. Changing indications and antenatal prognostic factors for ex-utero intrapartum treatment procedures. Prenat Diagn. 2022 Oct;42(11):1420-1428. doi: 10.1002/pd.6230. Epub 2022 Sep 9. PMID: 36045557.
- Shamshirsaz AA, Aalipour S, Erfani H, Nassr AA, Stewart KA, Kravitz ES, Rezaei A, Sanz Cortes M, Espinoza J, Belfort MA. Obstetric outcomes of ex-utero intrapartum treatment (EXIT). Prenat Diagn. 2019 Jul;39(8):643-646. doi: 10.1002/pd.5477. Epub 2019 May 29. PMID: 31093996.