More Information
Submitted: September 23, 2023 | Approved: October 03, 2023 | Published: October 04, 2023
How to cite this article: Weirich ML, Larkins CR, Craig WY, Meserve E, Febbraro T, et al. Age as a Predictor of Time to Response for Patients Undergoing Medical Management of Endometrial Cancer. Clin J Obstet Gynecol. 2023; 6: 150-159.
DOI: 10.29328/journal.cjog.1001144
Copyright License: © 2023 Weirich ML, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Endometrial cancer; Progesterone treatment; Endometrial intraepithelial neoplasia; Medical management
Age as a Predictor of Time to Response for Patients Undergoing Medical Management of Endometrial Cancer
M Larissa Weirich1*, Carolyn R Larkins2,3, Wendy Y Craig3,4, Emily Meserve5, Terri Febbraro6, Jason Lachance6 and Leslie S Bradford6
1Department of Obstetrics & Gynecology, Maine Medical Center, Portland, ME, USA
2Department of Obstetrics & Gynecology, University of Rochester, Rochester, NY, USA
3Tufts University School of Medicine, Boston, MA, USA
4Center for Clinical and Translational Sciences, MaineHealth Institute for Research, Scarborough, ME, USA
5Division of Anatomic Pathology, Spectrum Healthcare Partners, Portland, ME, USA
6Division of Gynecologic Oncology, MaineHealth/Maine Medical Center, Portland, ME, USA
*Address for Correspondence: M Larissa Weirich, MD, Department of Obstetrics and Gynecology, Maine Medical Center, Portland, ME, USA, Email: [email protected]
Objective: To explore the pathologic response rate to primary progesterone treatment in patients with Endometrial Intraepithelial Neoplasia (EIN) and early-stage endometrioid-type Endometrial Adenocarcinoma (EAC).
Methods: Retrospective chart data were collected for patients with either EIN or EAC receiving primary progesterone treatment between 2015 and 2022. The presence of complete or partial response, time to response, and other demographic and treatment factors were recorded to determine independent predictors of response to progestin treatment.
Results: In total, 112 women who were diagnosed with EIN or EAC were treated with upfront progestin therapy, of whom 79 had sufficient follow-up to assess response. Fifty patients (63%) responded, of whom 10 (20%) ultimately relapsed. Response was more robust among patients with EIN (79%, n = 33) compared with patients who had cancer (46%, n = 17). The median time to respond was 5.8 months overall. Diagnosis of EIN, younger age at diagnosis, and any pathologic evidence of progesterone effect were all predictors of treatment response. Younger patients had a significantly shorter time to partial or complete response with a median time to response of 5.9 months in patients ≤ 45 and 13.8 months in patients > 45.
Conclusion: Our study demonstrated a lower overall response rate (63%) than reported in previous studies, especially for patients with cancer (46%). Younger patients had a significantly shorter time to respond than older patients. Pathologic progesterone effect observed at any time during treatment was a significant predictor of treatment response regardless of diagnosis and could serve as an early predictor of response to therapy.
Endometrial carcinoma is the most common gynecologic malignancy in the US, with an estimated 65,950 cases diagnosed in 2022 [1]. Most cases are diagnosed at an early stage and carry a favorable prognosis. The most frequently diagnosed histologic subtype is endometrioid-type (type I) Endometrial Adenocarcinoma (EAC) comprising about 75% of cases. Endometrioid/endometrial intraepithelial neoplasia is the pathologic precursor to endometrioid-type endometrial adenocarcinoma. The 2014 WHO classification system of “Endometrioid” Intraepithelial Neoplasia (EIN) replaced the 1994 Complex Atypical Hyperplasia (CAH) terminology [2]. The American College of Obstetricians and Gynecologists (ACOG) uses the synonymous terminology “endometrial” intraepithelial neoplasia [3]. For the purposes of this study, we will consistently apply the ACOG term “Endometrial” Intraepithelial Neoplasia (EIN).
Approximately 67% of patients with type 1 endometrioid endometrial adenocarcinoma have a disease that is confined to the uterus at diagnosis, which is associated with an 80% - 90% 5-year survival rate depending on a variety of other factors [4]. The standard of care for endometrial carcinoma is surgical staging. There are some patients, however, who may not be deemed appropriate surgical candidates due to medical comorbidities or who would derive benefit from a fertility-sparing approach to treatment. One of the mainstays of non-surgical management is progestin therapy, given either orally or by placement of a levonorgestrel-releasing intrauterine device (L-IUD). The levonorgestrel-releasing IUD may be preferred for patients who have difficulty with daily medication adherence or who have other contraindications to systemic therapy. Response to progestin therapy is monitored by endometrial tissue sampling at regular intervals. The recommended duration of therapy is not yet well established but typically will continue until one of three outcomes occurs 1) completion of childbearing where appropriate, 2) progression of disease indicating nonresponse to therapy, 3) resolution/treatment of medical comorbidities enabling the patient to undergo definitive surgical therapy.
Consistent evidence thus far demonstrates response rates to progestin therapy of 80% - 90% for Endometrial Intraepithelial Neoplasia (EIN), while evidence for the treatment of endometrial carcinoma is less robust and suggests response rates of 50% - 70% [5-8]. Much of the quoted data is based on premenopausal women desiring future fertility. There is a paucity of data addressing the efficacy of medical management for endometrial cancer in the postmenopausal population undergoing non-surgical management due to medical comorbidities that preclude surgery, and further research is needed in this area. Other factors that must be further defined include the time to response, recurrence rates, length of therapy, and optimal interval of tissue sampling.
Among women living in Maine, cancer of the uterine corpus is the fourth most common malignancy diagnosed annually with an estimated 33.2 cases per 100,000 women per year [9]. Maine Medical Center is the sole gynecologic oncology practice in the state, thus providing a comprehensive and broad population for review. In this study, we explored the efficacy of non-surgical management of EAC and EIN at a single tertiary referral center. We assessed the outcomes of patients with EIN or grade 1 EAC who were managed with progestin therapy at our institution between October 2015 and June 2022.
All women ≥ 18 years with a diagnosis of EIN or grade 1 EAC who underwent primary progestin treatment with either systemic therapy or L-IUD within the Division of Gynecologic Oncology at MaineHealth/Maine Medical Center (Portland, ME, USA) during the study period 2015 - 2022 were potentially eligible for inclusion in the study. Institutional Review Board (IRB) approval was obtained to perform data analysis [Maine Medical Center Institutional Review Board, Portland, ME, #1811625-1]. Due to the retrospective nature of the study and the deidentified data, no direct patient care was affected in conducting this study. The study was deemed exempt from review by the IRB.
Patients were identified using billing data and ICD-10 diagnosis codes within the Electronic Medical Record (EMR), including the diagnosis codes of either endometrial intraepithelial neoplasia or malignant neoplasm of the endometrium. Search terms for progestin treatment included levonorgestrel-releasing IUD, megestrol acetate, medroxyprogesterone acetate, depot medroxyprogesterone, or a combination of these. Patients were excluded if they had histologic subtypes of endometrial cancer other than endometrioid, greater than clinical stage I disease, underwent primary surgical staging or debulking, were treated with primary radiation, or had a history of prior pelvic radiation therapy, or had inadequate follow up after initial consultation. For eligible patients, we collected demographic and clinical history data, as well as imaging studies, mode and timing of treatment, surgical procedures including hysterectomy during the study period, and pathology reports of all endometrial sampling within the study period including histology, grade of disease, and evidence of progestin effect. Those meeting all inclusion criteria were included for further analysis. All pathology data were reviewed and interpreted by fellowship-trained gynecologic pathologists at the treating institution.
Treatment response to progestin was defined according to methods described in previous studies [10-12]. Complete response was defined as the presence of benign endometrium without EIN or cancer. Partial response was defined as Endometrial Intraepithelial Neoplasia (EIN) in any patient who previously had endometrial cancer. No response or stable disease was defined as the persistence of the initial tissue diagnosis on a subsequent tissue sample (whether EIN or grade 1 endometrioid endometrial adenocarcinoma). Disease progression was defined as any cancer in a patient with a previous diagnosis of EIN, or evidence of higher-grade disease (grade 2 or above) in a patient with an initial diagnosis of grade 1 EAC. At the treating institution, evidence of progestin effect is often described in the pathology report if it is identified regardless of the final diagnosis. Evidence of progestin effect or no effect was also collected and used in the final analysis. The study was reviewed as exempt by the MaineHealth Institutional Review Board.
Statistical analysis
Characteristics of the study population were summarized using descriptive statistics, both overall and after stratification by pathologic diagnosis (EIN versus grade 1 EAC). Continuous data are shown as mean (standard deviation) or as median [interquartile range] as appropriate, and categorical data are shown as frequency (n, %). Differences in variables between subgroups were evaluated by t-tests or Mann-Whitney U tests for continuous data and by chi-square test or Fisher’s exact test for categorical data with corrections for multiple comparisons, as appropriate. Covariates that were significant (p < 0.1) in univariate analyses were entered into a logistic regression model to identify independent predictors of treatment response. We explored time to response or progression by survival analysis, using Kaplan Meier analysis with a log-rank test to evaluate differences between subgroups and Cox regression to adjust these differences for covariates (selected as described above). Significance was accepted at p < 0.05. All analyses were performed by the Navigation team at CORE using SPSS Statistical Software Version 28.
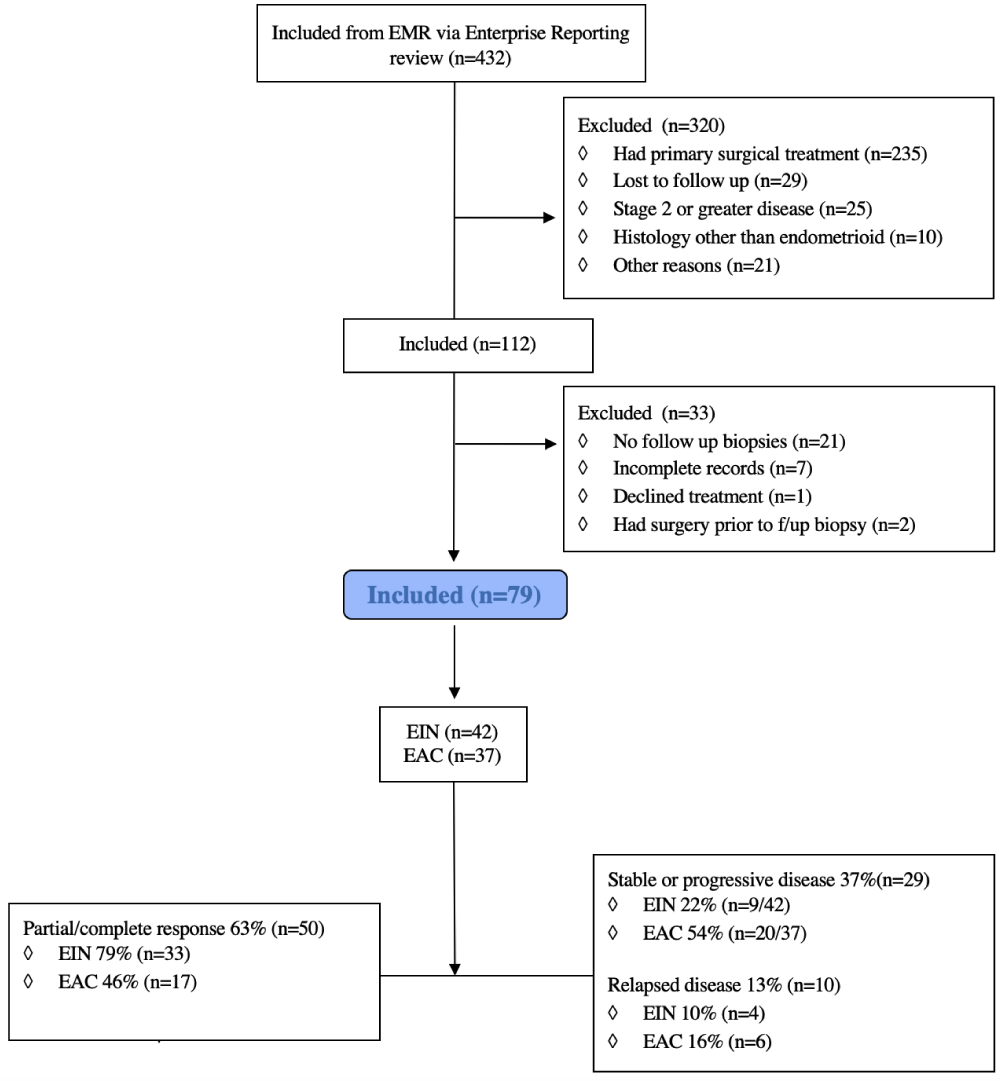
The selection process for patients included in this study is shown in the attached Consort Diagram 1. We identified 432 individuals for potential inclusion in the study. Of these, 320 patients were excluded as they underwent primary surgical staging (n = 235) or did not meet all the inclusion criteria (for example, progestin therapy had been started by the referring physician). Of the remaining 112 patients undergoing primary medical management, 79 had adequate follow-up biopsies to assess response and were included in the study.
Figure 1: Consort diagram.
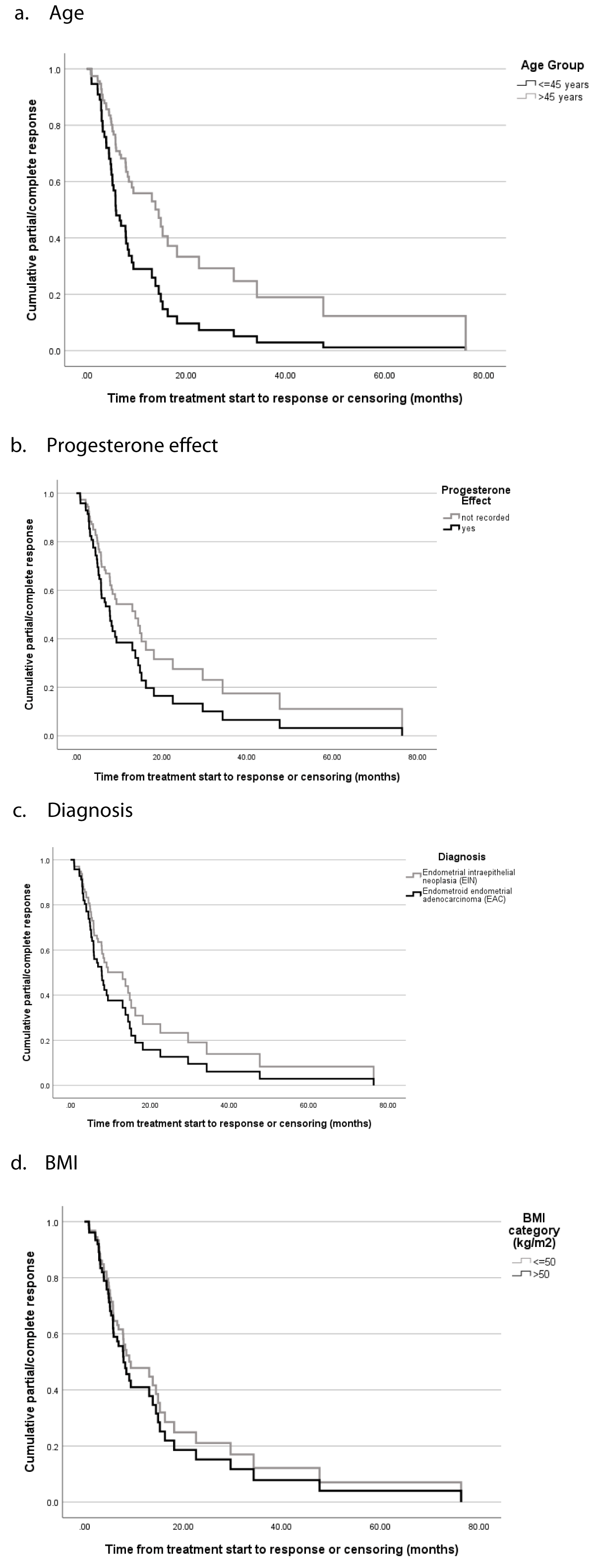
Figure 2: Time to response.
Table 1 [B] summarizes the demographic and clinical characteristics of the study group, stratified by diagnosis. 53% of patients (n = 42) were initially diagnosed with EIN while the remaining 47% (n = 37) had cancer at the time of initiation of medical management. Age, Body Mass Index (BMI), and parity were comparable between the two groups. The method of diagnosis included both endometrial biopsy (n = 41, 52%) and dilation and curettage (n = 38, 48%). Of the 41 patients diagnosed by endometrial biopsy, 18 (44%) subsequently underwent dilation and curettage. The procedure was generally performed at the time of L-IUD insertion rather than to confirm the diagnosis. One patient with a preoperative diagnosis of EIN was upgraded to a diagnosis of EAC after dilation and curettage. Most patients (n = 53, 67%) had one or more imaging studies prior to the treatment start date. These included transvaginal ultrasound (n = 43, 54%), computerized tomography (n = 9, 11%), or pelvic magnetic resonance imaging (n = 17, 22%). More women with endometrial cancer underwent advanced imaging including Computed Tomography (CT) or magnetic resonance imaging (MRI) (n = 19, 51%) compared with women who had EIN (n = 7, 17%).
| Table 1 [B]: Demographic characteristics of the study group. | |||
| Variable | Measurement1 Overall | EIN | EAC |
| N | 79 | 42 (53.2) | 37 (46.8) |
| Age (years) | 50.5 ± 17.8 | 44.8 ± 12.8 | 57.0 ± 20.4 |
| Body mass index (kg/m2) | 50.8 ± 14.3 | 52.6 ± 14.6 | 48.7 ± 13.9 |
| n | 77 | 41 | 37 |
| Race | |||
| White | 78 (98.7) | 42 (100.0)2 | 36 (97.3) |
| Asian | 1 (1.3) | 0 (0.0) | 1 (2.7) |
| Comorbidities3 | |||
| Cardiovascular disease | 9 (11.4) | 5 (11.9) | 4 (10.8) |
| Diabetes mellitus or insulin resistance | 28 (35.4) | 9 (21.4) | 19 (51.4) |
| Chronic obstructive pulmonary disease | 10 (12.7) | 5 (11.9) | 5 (13.5) |
| Polycystic ovary syndrome | 20 (25.3) | 12 (28.6) | 8 (21.6) |
| Concurrent malignancy4 | 1 (1.3) | 1 (2.4) | 0 (0.0) |
| None of the above | 7 (8.9) | 5 (11.9) | 2 (5.4) |
| Other | 49 (62.0) | 25 (59.5) | 24 (64.9) |
| Parous | 34/77 (44.2) | 18/41 (43.9) | 16/36 (44.4) |
| Number of children (among parous group) | |||
| 1 | 8/34 (23.5) | 7/18 (38.9) | 1/16 (6.2) |
| 2 | 18/34 (53.0) | 7/18 (38.9) | 11/16 (68.8) |
| ≥ 3 | 8/34 (23.5) | 4/18 (22.2) | 4/16 (25.0) |
| Family history of cancer, first degree relative | |||
| Uterine | 6 (7.6) | 5 (11.9) | 1 (2.7) |
| Ovarian or fallopian tube | 4 (5.1) | 1 (2.4) | 3 (8.1) |
| Breast | 9 (11.4) | 4 (9.5) | 5 (13.5) |
| Pancreatic/colorectal/other GI | 5 (6.3) | 1 (2.4) | 4 (10.8) |
| Other | 10 (12.7)5 | 6 (14.3) | 4 (10.8) |
| Family history of cancer, other relative | |||
| Uterine | 3 (3.8) | 2 (4.8) | 1 (2.7) |
| Ovarian or fallopian tube | 2 (2.5) | 1 (2.4) | 1 (2.7) |
| Breast | 13 (16.5) | 8 (19.0) | 5 (13.5) |
| Pancreatic/colorectal/other GI | 9 (11.4) | 5 (11.9) | 4 (10.8) |
| Other | 3 (3.8) | 2 (4.8) | 1 (2.7) |
| ECOGa performance status at diagnosis | |||
| 0 | 26 (32.9) | 11 (26.2) | 15 (40.5) |
| 1 | 8 (10.1) | 6 (14.3) | 2 (5.4) |
| 2 | 8 (10.1) | 2 (4.8) | 6 (16.2) |
| 3 | 5 (6.3) | 2 (4.8) | 3 (8.1) |
| 4 | 1 (1.3) | 0 (0.0) | 1 (2.7) |
| Not recorded | 31 (39.2) | 21 (50.0) | 10 (27.0) |
| aEastern Cooperative Oncology Group (ECOG). 1Data shown as frequency, n (%) or mean ± standard deviations. 2One person reported Hispanic ethnicity. 3In addition, patients overall reported: hypertension, 33 (42.3%); hyperlipidemia, 28 (35.9%); hypothyroidism, 12 (15.4%); sleep apnea, 17 (21.8); depression, 17 (21.8); anxiety, 5 (6.4%); asthma, 4 (5.1%); history of cancer, 3 (3.8%); renal disease, 5 (6.4%); and, other comorbidity, 20 (25.6%). 4Actively in treatment/ not in remission. 5One each of bladder cancer, lymphoma, thyroid cancer, cervical cancer and two of prostate cancer in the EIN group, one each of cervical/throat cancer, lung/bladder cancer, thyroid cancer, and glioblastoma in the EAC group. |
|||
Table 2 shows the distribution of treatment modalities and the indication for choosing progestin therapy [C]. The most frequent reasons cited by the treating clinician for the patient receiving medical rather than surgical therapy were desire for fertility preservation (n = 27, 34%) and surgical risk due to comorbidities (n = 54, 68%), with some citing both reasons. Approximately half of the patients (n = 38, 48%) were treated with L-IUD, and the others (n = 50, 63%) were treated with oral progestin, with the primary mode being megestrol acetate (n = 40, 51%). Nine patients were initiated on treatment with one modality (L-IUD or oral therapy) and a second therapy was subsequently added, reflecting the discrepancy in treatment numbers from the total of 79 patients. 16 patients (20%) received a combination therapy at the time of diagnosis with both intrauterine device and systemic therapy. The frequency of pathologic assessment during treatment was between 3 and 6 months for most patients (n = 73, 92%). 52 patients (66%) underwent at least 2 interval biopsies after treatment started, and 37 (n = 46%) had 3 or more biopsies. No pregnancies were reported after diagnosis.
| Table 2 [C]: Treatment characteristics. | |||
| Variable | Frequency, n (%) | ||
| Overall | EIN | EAC | |
| N | 79 | 42 | 37 |
| Mode of treatment | |||
| Oral medroxyprogesterone | 6 (7.6) | 5 (11.9) | 1 (2.7) |
| Oral megesterol acetate (megace) | 40 (50.6) | 21 (50.0) | 19 (51.4) |
| Levonorgestrel-releasing IUDa | 38 (48.1) | 23 (54.8) | 15 (40.5) |
| Combination (oral progestin with IUD) | 16 (20.3) | 8 (19.0) | 8 (21.6) |
| Other | 4 (5.1) | 2 (4.8)1 | 2 (5.4)2 |
| Number of treatment modes | |||
| 1 | 55 (69.6) | 25 (59.5) | 30 (81.1) |
| 2 | 23 (29.1) | 17 (40.5) | 6 (16.2) |
| 3 | 1 (1.3) | 0 (0.0) | 1 (2.7) |
| Intent of treatment (rather than surgery) | |||
| Fertility preservation | 27 (34.2) | 16 (38.1) | 11 (29.7) |
| Medical comorbidities/ surgical risk | 54 (68.4) | 27 (64.3) | 27 (73.0) |
| Patient declined surgery | 5 (6.3) | 3 (7.1) | 2 (5.4) |
| Cost | 1 (1.3) | 1 (2.4) | 0 (0.0) |
| aIntrauterine device. | |||
Detailed outcome data is reflected in Table 3 [D], both overall and after stratification by initial diagnosis. Complete or partial response was reported in 50 patients (63%), of whom 10 (20%) ultimately had a relapse of the disease. The remaining 29 (37%) had stable (n = 18, 23%) or progressive disease (n = 11, 14%). To identify potential predictors of treatment response, we first performed univariate analyses comparing demographic and clinical variables between subgroups that had either complete/partial response or no response/progression (Table 4a). The response was significantly more robust in patients with EIN (79%, n = 33) compared with patients who had cancer (46%, n = 17) (p = 0.006). Younger age (≤ 45 years, p < 0.001) and any pathologic evidence of progestin effect (p < 0.001) were also significantly associated with treatment response. However, after adjusting for covariates (Table 4b) only the presence of progestin effect was independently associated with a response to treatment [adjusted OR (95% CI), 3.7 (1.2 - 11.8), p = 0.026]. Patients aged ≤ 45 were more likely to have evidence of progestin response compared with patients > 45 (p < 0.001). However, age was not independently predictive of a response to treatment (p = 0.09).
| Table 3 [D]: Outcomes after treatment. | |||
| Variable | Frequency, n (%) or Median [interquartile range] | ||
| Overall | EIN | EAC | |
| Treatment response | |||
| N | 79 | 42 | 37 |
| Partial/Complete1 | 50 (63.3) | 33 (78.6) | 17 (46.0) |
| Stable/Progression | 29 (36.7) | 9 (21.4) | 20 (54.0) |
| Change in BMI since diagnosis (kg/m2) | 0 [-7.2- +1.1] | -1.0 | [-3.7 - +1.0] |
| n | 76 | 41 (-38- +9) | 35 (-15.6 to +14) |
| Patient had hysterectomy | 34/77 (44.2) | 18/40 (45.0) | 16 (43.2) |
| Indication for hysterectomy | |||
| Disease progression | 12 (35.3) | 6 (33.3) | 6 (37.5) |
| Stable or unresponsive disease | 12 (35.3) | 3 (16.7) | 9 (56.3) |
| Patient request | 4 (11.8) | 4 (22.2) | 0 (0.0) |
| Improvement in performance status | 5 (14.7) | 5 (27.8) | 0 (0.0) |
| Not specified | 1 (2.9) | 0 (0.0) | 1 (6.3) |
| Pathologic diagnosis of hysterectomy tissue | |||
| N | 34 | 18 | 16 |
| Endometrial intra-epithelial neoplasia | 4 (11.8) | 4 (22.2) | 0 (0.0) |
| Endometrial cancer | 22 (64.7) | 6 (33.3) | 16 (100.0) |
| EIN with foci of endometrial cancer | 1 (2.9) | 1 (5.6) | 0 (0.0) |
| Hyperplasia without neoplasia | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Benign endometrium | 7 (20.6) | 7 (38.9) | 0 (100.0) |
| Grade and stage of endometrial cancer | |||
| N | 22 | 6 | 16 |
| Grade | |||
| 1 | 17 (77.3) | 6 (100.0) | 11 (68.8) |
| 2 | 4 (18.2) | 0 (0.0) | 4 (25.0) |
| 3 | 1 (4.5) | 0 (0.0) | 1 (6.2) |
| Stage | |||
| IA | 13 (59.1) | 6 (100.0) | 7 (43.8) |
| IB | 3 (13.6) | 0 (0.0) | 3 (18.8) |
| II or higher | 6 (27.3) | 0 (0.0) | 6 (37.5) |
| Vital status at end of follow up period | |||
| N | 79 | 42 | 37 |
| Deceased | 9 (11.4) | 1 (2.4) | 8 (21.6) |
| Alive | 70 (88.6) | 41 (97.6) | 29 (78.4) |
| No evidence of disease | 43 | 27 | 16 |
| Alive with disease | 9 | 5 | 4 |
| Table 4 [E]: Identifying predictors of treatment response. | |||
| a) Variables associated with treatment response. | |||
| Variable | Treatment response1 Partial/Complete2 | Stable/Progression | p - value |
| N | 50 | 29 | |
| Diagnosis | |||
| EIN | 33 (66.0) | 9 (31.0) | 0.0063 |
| EAC | 17 (34.0) | 20 (69.0) | |
| Age (years) | 45.0 ± 14.6 | 60.0 ± 19.0 | < 0.0014 |
| Body mass index (kg/m2) | 52.7 ± 14.3 (n = 49) | 47.4 ± 13.9 (n = 28) | 0.064 |
| Parous | 23/49 (46.9) | 11/28 (39.3) | 0.683 |
| Comorbidities | |||
| Cardiovascular disease | 3 (6.0) | 6 (20.7) | 0.075 |
| Diabetes/insulin resistance | 16 (32.0) | 12 (41.4) | 0.553 |
| COPDa | 4 (8.0) | 6 (20.7) | 0.165 |
| PCOSb | 15 (30.0) | 5 (17.2) | 0.323 |
| Concurrent malignancy | 0 (0.0) | 1 (3.4) | 0.375 |
| Progesterone effect6 | 40 (80.0) | 11 (37.9) | < 0.0013 |
| DNA mismatch repair (MMR) protein status | |||
| Proficient | 7/49 (14.3) | 11 (37.9) | 0.135,7 |
| Deficient | 0/49 (0.0) | 6 (20.7) | |
| Not specified | 42/49 (85.7) | 12 (41.4) | |
| 4b) Independent predictors of treatment response. | |||||
| Variable | Reference group | Odds ratio for partial/complete response (95% CI) | |||
| Unadjusted | p - value | Adjusted | p - value8 | ||
| EIN | EAC | 4.3 (1.6 - 11.5) | 0.003 | 2.1 (0.7 - 6.5) | 0.12 |
| Progesterone effect9 | None | 6.5 (2.4 - 18.2) | < 0.001 | 3.7 (1.2 - 11.8) | 0.026 |
| Age | - | 0.95 (0.92 - 0.98) | < 0.001 | 0.97 (0.94 - 1.01) | 0.09 |
| BMI | - | 1.03 (0.99 - 1.06) | 0.38 | 1.01 (0.98 - 1.06) | 0.49 |
| aChronic obstructive pulmonary disease. bPolycystic ovary syndrome. 1Data shown as frequency, n (%) or mean ± standard deviation. 2Includes 10 patients who ultimately relapsed. 3Chi square test with continuity correction. 4two-sided t test. 5Fisher’s exact test. 6Observed at most recent biopsy. 7Analysis excludes those with MMR status unspecified. 8Variables that were significant (p ≤ 0.1) in univariate analysis (Table 9) were entered into a logistic regression model to identify significant independent predictors of treatment response. Treatment response was defined as a binary variable (partial/complete, including those who eventually relapsed vs stable/progression). n = 77 cases had complete data for all variables and were included in the analysis. 9Observed at any point during treatment. |
|||||
Among those with treatment responses, the median time to respond was 5.8 months overall [3.2 - 10.3]. The time to progression of disease was 8.0 months overall [range 3.6 - 8.7]. Among patients who relapsed, the median time to relapse was 30.4 months [range 15.9 - 39.6]. There was no difference in response to treatment based on the mode of progestin therapy (intrauterine versus systemic). Table 5 [F] summarizes the findings of survival analysis. Younger patients (aged ≤ 45 years) had a significantly shorter time to partial or complete response compared with those aged > 45 years (5.9 months [range 2.7 - 9.1] versus 13.8 months [range 6.5 - 21.2], respectively, p = 0.012). After adjusting for covariates [F, Table 5b], age ≤ 45 years remained a significant predictor of time to response (hazard ratio (95% confidence interval), 2.13 (1.07 - 4.23), p = 0.03).
| Table 5 [F]: Predictors of partial/complete treatment response and time to response. | ||||
| a) Variables associated with time from treatment start to partial/complete response. | ||||
| Response | Time to response or censor (months)3 | |||
| Progestin effect2 | n | n (%) | Median (95% CI) | p - value4 |
| Yes | 51 | 40 (78.4) | 7.8 (5.5 - 10.1) | 0.06 |
| No | 28 | 10 (35.7) | 13.8 (7.2 - 20.4) | |
| Diagnosis | ||||
| EIN | 42 | 33 (78.6) | 7.8 (5.0 - 10.6) | 0.64 |
| EAC | 37 | 17 (45.9) | 13.8 (3.0 - 24.6) | |
| Age group (years) | ||||
| ≤ 45 | 34 | 26 (76.5) | 5.9 (2.7 - 9.1) | 0.012 |
| > 45 | 45 | 24 (53.3) | 13.8 (6.5 - 21.2) | |
| BMI group (kg/m2) | ||||
| ≤ 50 | 38 | 21 (55.2) | 9.1 (7.2 - 10.9) | 0.99 |
| > 50 | 39 | 28 (71.8) | 7.8 (4.3 - 11.2) | |
| 5b) Independent predictors of time to partial/complete response. | |||||
| Variable | Reference | Unadjusted | Adjusted | ||
| HR (95% CI) | p - value5 | HR (95% CI) | p - value5 | ||
| Progestin effect | no effect | 1.92 (0.95 - 3.86) | 0.07 | 1.56 (0.71 - 3.4) | 0.27 |
| EIN | EAC | 1.16 (0.63 - 2.14) | 0.64 | 0.71 (0.36 - 1.40) | 0.32 |
| Age ≤ 45 years | Age > 45 years | 2.05 (0.96 - 0.99) | 0.014 | 2.13 (1.07 - 4.23) | 0.03 |
| BMI ≤ 50 kg/m2 | BMI > 50 kg/m2. | 1.00 (0.56 - 1.81) | 0.99 | 0.82 (0.44 - 1.56) | 0.56 |
| 1Partial or complete response. 2Time from diagnosis to partial/complete response or censoring (progression, most recent biopsy, or hysterectomy if earlier); median time estimated by Kaplan Meyer survival analysis. 3Log rank test. 4Cox regression; 40 cases with an event and 37 cases that were censored were included in the adjusted analysis. Hazard ratio reflects “hazard” for having a complete/partial response. Variables were entered into the model if they had a significant (p < 0.1) association with partial/complete response in univariate analysis (Table 4). |
|||||
During the treatment period, a total of 34 patients (43%) ultimately underwent hysterectomy. For most patients, the indication for hysterectomy was disease progression or inadequate response to progestin therapy (n = 24, 71%). Five patients (15%) had surgery because of an improvement in their health or performance status, generally related to weight loss and/or improved glycemic control. The remaining five patients had various reasons for undergoing surgery, including patient requests and changes in desire for fertility preservation. Within this cohort of 34 patients, 18 patients (n = 53%) had a preoperative diagnosis of EIN. Six of these patients (33%) had cancer detected on the final pathology. In all of these cases, the cancer was grade 1 and International Federation of Gynecology and Obstetrics (FIGO) Stage IA. Of the 16 patients with a preoperative diagnosis of cancer, 6 patients with presumed Stage I disease were diagnosed with Stage II or higher disease on final pathology, thus requiring adjuvant therapy.
Many questions remain regarding optimal management for patients with early-stage endometrial cancer being treated with progestin therapy, despite the research thus far on this subject. Most studies suggest complete response rates, typically defined by pathologic regression of disease on endometrial biopsy, ranging from 50% - 70% [7,13-18]. The current literature suggests higher response rates for women with EIN compared with those who have cancer. Much of the data thus far, however, focuses on premenopausal patients who desire fertility preservation. More research is needed in the postmenopausal patient population and among those who are deemed too medically unwell to undergo surgery. A retrospective study by Baker, et al. [10] specifically examined response rates in postmenopausal women who were poor surgical candidates. Baker, et al. demonstrated a complete response rate of 50% overall for EIN and EAC, and patients with EIN were not more likely to achieve a complete response. This warrants further study, as it suggests that factors other than grade of disease play a role in determining progestin response. Our study included more postmenopausal patients with medical comorbidities than have been included in previous studies with the exception of the Baker et al. study. This may have contributed to the lower response rate seen in our patient population, which warrants further investigation.
Our study included patients undergoing medical treatment for endometrial intraepithelial neoplasia and low-grade endometrioid-type endometrial adenocarcinoma. With an overall response rate of 63% including partial and complete response, our study demonstrated a lower-than-expected response based on what has been reported in previous studies. Age was a significant predictor of time to progesterone response, with younger patients having a shorter time to respond. Evidence of progestin effect was independently predictive of a response to therapy. As has been demonstrated in past studies, patients with EIN were more likely to respond to treatment (78%) than patients with cancer (46%). However, the diagnosis was not independently predictive of treatment response, which suggests that other factors may be more important in determining a response to therapy.
One explanation for this is the potential impact of BMI. Obesity is a risk factor for the development of endometrial neoplasia due to the endometrium’s increased exposure to unopposed estrogen. While past studies have shown a difference in treatment response associated with BMI [19], we did not find a statistically significant difference between responders and non-responders when stratified by BMI (p = 0.49). Weight loss reduces the risk of developing endometrial neoplasia and improves overall survival in patients with endometrial cancer [20], but its effect on response to progesterone treatment is still uncertain based on available evidence. The feMME trial showed a trend toward improved response in patients treated with LNG-IUD who lost weight compared with those who did not, but the study was not powered to detect significance between these groups [21]. In our study, patients who lost weight during the treatment period did not have improved response rates compared with patients who remained at stable weight or gained weight. The cohort of patients with this information recorded may be too small to detect a significant effect of change in BMI, and more research with a larger cohort would be beneficial to further evaluate this.
In this study, we examined the response to progestin therapy in premenopausal patients who desire fertility preservation and older patients who were too medically unwell to undergo surgery. The inclusion of both cohorts provides the unique opportunity to compare response rates in these distinct patient populations. The goals of treatment are often different for these patient groups. For example, women who desire future fertility may plan to undergo a short duration of treatment prior to pregnancy, with the aim of definitive surgical therapy when childbearing is completed. For older medically complex patients, planned treatment duration may be quite variable and extend until the end of life if possible. Our study demonstrated no difference in treatment response between older and younger patients when controlling for diagnosis. A unique finding in our study is that there was a significant association between age and time to treatment response, with younger patients responding more quickly to therapy. This could be an important factor in counseling patients when considering alternate treatments due to lack of response. It could also be beneficial for patients desiring future fertility to undergo a shorter treatment period.
The recurrence rate for patients who initially respond has also been documented by previous studies. This is especially relevant in determining the appropriate interval for tissue sampling for these patients, and for risk stratifying patients to different treatment modalities. Recurrence rates have been reported between 22% and 47% [14,7,13,22,10]. This is usually defined as women who initially demonstrated complete pathologic response, and on subsequent endometrial sampling during the study period had evidence of recurrent disease or neoplasia. In our study, of the initial cohort who showed response to therapy (n = 50), 10 patients ultimately had relapse or progression of disease. Median time to relapse was over 2 years, suggesting that recurrence of disease in this cohort may be representative of the duration of treatment rather than a true failure of medical therapy. Diagnosis (EIN or EAC) did not differ between patients who experienced recurrence, which again suggests that other factors are important in determining treatment response.
During the treatment period we observed that 24 patients (30%) underwent hysterectomy due to disease progression or lack of treatment response. On final pathology, 6 of these patients were upstaged and required adjuvant treatment for stage II or greater disease. This area requires further investigation to improve medical management for the patients who desire fertility or for whom surgery is too morbid. Multiple studies have examined the use of metformin in addition to progestin for treatment of endometrial cancer with conflicting results [23-25]. These studies are predominantly conducted in premenopausal women desiring fertility preservation. It is difficult to study the effect of metformin on treatment response in the older medically unwell population, as many of these patients are actively undergoing treatment for diabetes. It has also been hypothesized that addition of mTOR inhibitors could enhance response to progestin therapy, as activation of the mTOR pathway can be associated with resistance to hormonal therapy. This an area of ongoing research, with trials currently investigating the use of mTOR inhibitors (everolimus) in combination with progestin to treat early-stage endometrial cancer.
The strengths of our study include a consistent approach to pathologic assessment and reporting of progestin response with a relatively small number of fellowship-trained gynecologic pathologists examining samples, which improves reliability. Additionally, the study was performed at a single institution and all the treatment and follow up strategies are consistent, which makes accurate and thorough data collection possible. Our study is also unique in that prior investigations have focused on a younger cohort pursuing fertility-sparing options, whereas this cohort includes patients deemed to be poor surgical candidates.
There are limitations to this study, many of which are inherent to any retrospective review. The retrospective nature of this investigation and the small sample size preclude our ability to draw conclusions regarding hormonal regimen (e.g., IUD vs. oral vs. IUD plus oral) as well our ability to draw conclusions regarding impact of change in BMI over the time course of treatment. Additionally, the predominately white demographic that makes up the population in Maine (94% according to the 2021 Census) could limit the generalizability of the results. This is reflected in the limited racial diversity of the study population (98.7% white).
In summary, our study demonstrated a lower overall response rate than previously reported in the literature, and this was more pronounced for patients with cancer, with an overall response rate of 46%. Younger patients had a significantly shorter time to response than older patients and pathologic progesterone effect observed at any time during treatment was a significant predictor of treatment response regardless of diagnosis and could serve as an early predictor of response to therapy. We did not find a statistically significant difference between responders and non-responders when stratified by age, BMI at time of diagnosis, or weight loss while undergoing hormonal therapy, but acknowledge that other factors and molecular pathways may play a role determining a response to therapy. Including women who are postmenopausal and deemed poor surgical candidates is crucial to improving outcomes for patients undergoing non-surgical management of this disease.
This work was supported in part by the Northern New England Clinical and Translational Research grant U54GM115516.
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022 Jan;72(1):7-33. doi: 10.3322/caac.21708. Epub 2022 Jan 12. PMID: 35020204.
- Höhn AK, Brambs CE, Hiller GGR, May D, Schmoeckel E, Horn LC. 2020 WHO Classification of Female Genital Tumors. Geburtshilfe Frauenheilkd. 2021 Oct;81(10):1145-1153. doi: 10.1055/a-1545-4279. Epub 2021 Oct 6. PMID: 34629493; PMCID: PMC8494521.
- The American College of Obstetricians and Gynecologists Committee Opinion no. 631. Endometrial intraepithelial neoplasia. Obstet Gynecol. 2015 May;125(5):1272-1278. doi: 10.1097/01.AOG.0000465189.50026.20. PMID: 25932867.
- Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, Chon HS, Chu C, Cohn D, Crispens MA, Damast S, Dorigo O, Eifel PJ, Fisher CM, Frederick P, Gaffney DK, George S, Han E, Higgins S, Huh WK, Lurain JR 3rd, Mariani A, Mutch D, Nagel C, Nekhlyudov L, Fader AN, Remmenga SW, Reynolds RK, Tillmanns T, Ueda S, Wyse E, Yashar CM, McMillian NR, Scavone JL. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018 Feb;16(2):170-199. doi: 10.6004/jnccn.2018.0006. PMID: 29439178.
- Affinito P, Di Carlo C, Di Mauro P, Napolitano V, Nappi C. Endometrial hyperplasia: efficacy of a new treatment with a vaginal cream containing natural micronized progesterone. Maturitas. 1994 Dec;20(2-3):191-8. doi: 10.1016/0378-5122(94)90016-7. PMID: 7715472.
- Varma R, Soneja H, Bhatia K, Ganesan R, Rollason T, Clark TJ, Gupta JK. The effectiveness of a levonorgestrel-releasing intrauterine system (LNG-IUS) in the treatment of endometrial hyperplasia--a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol. 2008 Aug;139(2):169-75. doi: 10.1016/j.ejogrb.2008.02.022. Epub 2008 Apr 28. PMID: 18440693.
- Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, Nakanishi T, Sasaki H, Saji F, Iwasaka T, Hatae M, Kodama S, Saito T, Terakawa N, Yaegashi N, Hiura M, Sakamoto A, Tsuda H, Fukunaga M, Kamura T. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007 Jul 1;25(19):2798-803. doi: 10.1200/JCO.2006.08.8344. PMID: 17602085.
- Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med. 2010 Jan;28(1):81-90. doi: 10.1055/s-0029-1242998. Epub 2010 Jan 26. PMID: 20104432; PMCID: PMC4767501.
- Maine Cancer Registry. “2022 Maine Cancer Snapshot.” Maine Cancer Registry, based on November 2021 NPCR-CSS data submission: Maine Department of Health and Human Services, Maine Center for Disease Control and Prevention.
- Baker WD, Pierce SR, Mills AM, Gehrig PA, Duska LR. Nonoperative management of atypical endometrial hyperplasia and grade 1 endometrial cancer with the levonorgestrel intrauterine device in medically ill post-menopausal women. Gynecol Oncol. 2017 Jul;146(1):34-38. doi: 10.1016/j.ygyno.2017.04.006. Epub 2017 Apr 18. PMID: 28427775.
- Hubbs JL, Saig RM, Abaid LN, Bae-Jump VL, Gehrig PA. Systemic and local hormone therapy for endometrial hyperplasia and early adenocarcinoma. Obstet Gynecol. 2013 Jun;121(6):1172-1180. doi: 10.1097/AOG.0b013e31828d6186. PMID: 23812449.
- Westin SN, Fellman B, Sun CC, Broaddus RR, Woodall ML, Pal N, Urbauer DL, Ramondetta LM, Schmeler KM, Soliman PT, Fleming ND, Burzawa JK, Nick AM, Milbourne AM, Yuan Y, Lu KH, Bodurka DC, Coleman RL, Yates MS. Prospective phase II trial of levonorgestrel intrauterine device: nonsurgical approach for complex atypical hyperplasia and early-stage endometrial cancer. Am J Obstet Gynecol. 2021 Feb;224(2):191.e1-191.e15. doi: 10.1016/j.ajog.2020.08.032. Epub 2020 Aug 15. PMID: 32805208; PMCID: PMC7855308.
- Chiva L, Lapuente F, González-Cortijo L, Carballo N, García JF, Rojo A, Gonzalez-Martín A. Sparing fertility in young patients with endometrial cancer. Gynecol Oncol. 2008 Nov;111(2 Suppl):S101-4. doi: 10.1016/j.ygyno.2008.07.056. Epub 2008 Sep 18. PMID: 18804267.
- Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol. 2004 Oct;95(1):133-8. doi: 10.1016/j.ygyno.2004.06.045. PMID: 15385122.
- Montz FJ, Bristow RE, Bovicelli A, Tomacruz R, Kurman RJ. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002 Apr;186(4):651-7. doi: 10.1067/mob.2002.122130. PMID: 11967486.
- Wheeler DT, Bristow RE, Kurman RJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007 Jul;31(7):988-98. doi: 10.1097/PAS.0b013e31802d68ce. PMID: 17592264.
- Baker W, Pelkofski E, Duska L. Levonorgestrel intrauterine device (IUD) treatment of complex atypical hyperplasia and grade 1 endometrioid endometrial cancer in postmenopausal women. Gynecologic Oncology. 2014; 133:158.
- Pal N, Broaddus RR, Urbauer DL, Balakrishnan N, Milbourne A, Schmeler KM, Meyer LA, Soliman PT, Lu KH, Ramirez PT, Ramondetta L, Bodurka DC, Westin SN. Treatment of Low-Risk Endometrial Cancer and Complex Atypical Hyperplasia With the Levonorgestrel-Releasing Intrauterine Device. Obstet Gynecol. 2018 Jan;131(1):109-116. doi: 10.1097/AOG.0000000000002390. PMID: 29215513; PMCID: PMC5739955.
- Li M, Guo T, Cui R, Feng Y, Bai H, Zhang Z. Weight control is vital for patients with early-stage endometrial cancer or complex atypical hyperplasia who have received progestin therapy to spare fertility: a systematic review and meta-analysis. Cancer Manag Res. 2019 May 6;11:4005-4021. doi: 10.2147/CMAR.S194607. PMID: 31190979; PMCID: PMC6512613.
- Kitson S, Ryan N, MacKintosh ML, Edmondson R, Duffy JM, Crosbie EJ. Interventions for weight reduction in obesity to improve survival in women with endometrial cancer. Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD012513. doi: 10.1002/14651858.CD012513.pub2. Update in: Cochrane Database Syst Rev. 2023 Mar 27;3:CD012513. PMID: 29388687; PMCID: PMC6491136.
- Janda M, Robledo KP, Gebski V, Armes JE, Alizart M, Cummings M, Chen C, Leung Y, Sykes P, McNally O, Oehler MK, Walker G, Garrett A, Tang A, Land R, Nicklin JL, Chetty N, Perrin LC, Hoet G, Sowden K, Eva L, Tristram A, Obermair A. Complete pathological response following levonorgestrel intrauterine device in clinically stage 1 endometrial adenocarcinoma: Results of a randomized clinical trial. Gynecol Oncol. 2021 Apr;161(1):143-151. doi: 10.1016/j.ygyno.2021.01.029. Erratum in: Gynecol Oncol. 2021 Aug;162(2):526. PMID: 33762086.
- Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012 May;125(2):477-82. doi: 10.1016/j.ygyno.2012.01.003. Epub 2012 Jan 11. PMID: 22245711.
- Mitsuhashi A, Habu Y, Kobayashi T, Kawarai Y, Ishikawa H, Usui H, Shozu M. Long-term outcomes of progestin plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer patients. J Gynecol Oncol. 2019 Nov;30(6):e90. doi: 10.3802/jgo.2019.30.e90. PMID: 31576686; PMCID: PMC6779615.
- Yang BY, Gulinazi Y, Du Y, Ning CC, Cheng YL, Shan WW, Luo XZ, Zhang HW, Zhu Q, Ma FH, Liu J, Sun L, Yu M, Guan J, Chen XJ. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: a randomised controlled trial. BJOG. 2020 Jun;127(7):848-857. doi: 10.1111/1471-0528.16108. Epub 2020 Feb 16. PMID: 31961463.
- Acosta-Torres S, Murdock T, Matsuno R, Beavis AL, Stone RL, Wethington SL, Levinson K, Grumbine F, Ferriss JS, Tanner EJ, Fader AN. The addition of metformin to progestin therapy in the fertility-sparing treatment of women with atypical hyperplasia/endometrial intraepithelial neoplasia or endometrial cancer: Little impact on response and low live-birth rates. Gynecol Oncol. 2020 May;157(2):348-356. doi: 10.1016/j.ygyno.2020.02.008. Epub 2020 Feb 19. PMID: 32085863.