More Information
Submitted: September 14, 2023 | Approved: September 28, 2023 | Published: September 29, 2023
How to cite this article: Suliman AA, Ibrahim Ahmed HS, Adam Hammad KM, Youssef Alsiddig IJ, Elamin Abdelgader MA, et al. Ectopic Pregnancy Risk Factors Presentation and Management Outcomes. Clin J Obstet Gynecol. 2023; 6: 143-149.
DOI: 10.29328/journal.cjog.1001143
Copyright License: © 2023 Suliman AA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Ectopic; Pregnancy; Risk; Presentation; Management; Outcomes
Ectopic Pregnancy Risk Factors Presentation and Management Outcomes
Awadalla Abdelwahid Suliman1*, Hajar Suliman Ibrahim Ahmed1, Kabbashi Mohammed Adam Hammad1, Ibtehal Jaffer Youssef Alsiddig2, Mohamed Abdalla Elamin Abdelgader3, Abdallah Omer Elzein Elhag4 and Safa Mohamed Ibrahim5
11Assistant Professor of Obstetrics and Gynecology, Faculty of Medicine, Al Neelain University, Sudan
2Specialist of Obstetrical & Gynecology, Sudan Medical Specialization Board, Sudan
3Internal Medicine Specialist, Sudan Medical Specialization Board, Sudan
4Consultant of Obstetrics and Gynecology, Sudan Medical Specialization Board, Sudan
5Consultant Physician Sudan Medical Specialization Board, Sudan
*Address for Correspondence: Awadalla Abdelwahid Suliman, Consultant of Obstetrics and Gynecology, Faculty of Medicine, Al Neelain University, Khartoum, Sudan, Email: [email protected]
Background: Ectopic pregnancy (EP) is a common and serious early pregnancy problem with a significant morbidity rate and the potential for maternal death. Women commonly present with minimal vaginal bleeding and abdominal pain.
Objective: The main objective of the study was to evaluate the risk factors, clinical presentation, sites, and management outcomes of ectopic pregnancies.
Methodology: It was a prospective descriptive, cross-sectional hospital-based study conducted at Bashair Teaching Hospital during the period January 2021–June 2021.
An interview questionnaire was used, and eighty-two (82) women were included after informed consent. Demographic and clinical data concerning personal history, symptoms of presentation, risk, site, and type of management were recorded.
Results: Ectopic pregnancy incidence was 2% and most risk factors were infection 29.3%, surgery 15.9%, miscarriage 13.4%, infertility 12.2%, tubal surgery 4.9%, previous ectopic pregnancy 4.9%, intrauterine contraceptive device (IUCD) 3.6%, and tubal ligation 2.4%. Women presented with bleeding and abdominal pain at 47.5%, bleeding at 18.3%, abdominal pain at 9.7%, and shock at 8.5%.
The sites are ampullary (57.3%), fimbria (9.7%), interstitial (8.5%), isthmus (8.5%), ovarian (7.3%), cervical (4.8%), and abdominal (3.6%).
Surgical management was 93.9%, medical and surgical management was 3.6% and medical management was 2.4%. A blood transfusion was received at 37.8%.
Conclusion: The study concluded that women of reproductive age are at risk of ectopic pregnancy, so healthcare providers and doctors should have a high index of suspicion, prompt diagnosis, and intervention for ectopic pregnancy. Assessment of women at risk factors and modifications will reduce incidence.
Ectopic pregnancy is defined as a pregnancy that occurs outside of the uterine cavity. The most common site of ectopic pregnancy is the fallopian tube, this type of ectopic pregnancy is called tubal pregnancy.
Sometimes, an ectopic pregnancy occurs in other areas of the body, such as the ovary, abdominal cavity, or the lower part of the uterus (cervix), which connects to the vagina.
Most cases of tubal ectopic pregnancy (EP) that are detected early can be treated successfully either with minimally invasive surgery or with medical management using methotrexate [1].
Risk Factors for Ectopic Pregnancy are age of more than 35 years, cigarette smoking, documented fallopian tube pathology, history of Infertility, pelvic inflammatory disease, pregnancy while an intrauterine device is in place, pelvic surgery, previous ectopic pregnancy, previous fallopian tube surgery and in vitro fertilization [2,3].
Sites of ectopic pregnancy are, tubal 95%, interstitial 2%-4%, Ovarian < 3%, heterotopic 1%-3%, abdominal, 1%cesarean scar, < 1% and cervical < 1% [4-6].
Ectopic pregnancy should be considered in any pregnant woman with amenorrhea, vaginal bleeding, or lower abdominal pain when intrauterine pregnancy has not yet been diagnosed vaginal bleeding in women with ectopic pregnancy is due to the sloughing of decidual endometrium which can range from spotting to menstruation-equivalent levels [2,7,].
Diagnosis of ectopic pregnancy by serum Beta-human chorionic gonadotrophin (β-hCG) levels in correlation with transvaginal ultrasound (TVUS) or transabdominal ultrasound (TAUS) findings. TVUS is more accurate and sensitive than TAUS in diagnosing early EP [8]. Specifically, three-dimensional TVUS combined with color Doppler US was shown to be more effective than conventional 3-dimensional ultrasound (3D-US) for the diagnosis of early cesarean scar pregnancy [9].
Ectopic pregnancy can be managed with conservative, medical or surgical treatment according to presentation and the woman's general conditions and it depends on EP location, pregnancy timeline, and gestational sac size.
Expectant management is the most conservative approach for the treatment of EPs. This method can be considered for patients with decreasing or plateaued β-hCG levels.
Medical management by intramuscular methotrexate (MTX) injection is the current standard for medical management of EPs [2]. It has some contraindications which include hemodynamic instability, anemia, leukopenia, thrombocytopenia, pelvic pain or hemoperitoneum [10], indicative of EP rupture, renal or hepatic insufficiency, pulmonary disease, active peptic ulcer disease, coinciding IUP, breastfeeding, fetal cardiac activity, serum β-hCG levels > 5000 mIU/mL, or EP > 4 cm in diameter.
Surgical management is indicated in patients exhibiting MTX contraindications. MTX is administered in single, double, or multi-dose regimens. Double dose protocol is more effective than single dose [11].
Surgical management Salpingostomy and salpingectomy are the two common approaches for surgical management of Eps, which are done by opened laparotomy or laparoscopy [12].
The treatment in developing countries, surgery remains the mainstay of treatment, mostly performed in an emergency, with frequent tubal rupture and hemoperitoneum [13]. If the ectopic pregnancy has been diagnosed, the patient is hemodynamically stable, and the affected fallopian tube has not ruptured, treatment options include medical management with intramuscular methotrexate or surgical management with salpingostomy (removal of the ectopic pregnancy while leaving the fallopian tube in place) or salpingectomy (removal of part or all of the affected fallopian tube) [7].
The decision to manage the ectopic pregnancy medically or surgically should be informed by individual patient factors and preferences, clinical findings, ultrasound findings, and β-hCG levels. Expectant management is rare but can be considered with close follow-up for patients with suspected ectopic pregnancy who are asymptomatic and have very low β-hCG levels that continue to decrease [7,14].
It was a descriptive prospective cross-sectional hospital-based study conducted at Bashair Teaching Hospital during the period January 2021 – June 2021.
The Study population that was included all women diagnosed with ectopic pregnancy who came during the study period to the gynecology clinic and emergency room, and they agreed to participate in the study. About 82 women who were diagnosed with ectopic pregnancy were included.
Data was collected by direct interview by using a well-structured questionnaire. The participants were interviewed about age, education, gestational age, parity, risk factors for ectopic pregnancy, type of ectopic, management type, hospital stay, Anti-D administration, and blood transfusion.
Statistical analysis was performed via SPSS software (SPSS, Chicago, IL, USA). Continuous variables were compared using the student’s t - test (for paired data) or the Mann–Whitney U test for nonparametric data. For categorical data, a comparison was done using the Chi-square test (X2) or Fisher’s exact test when appropriate. A p - value of < 0.05 was considered statistically significant.
Ethical considerations
Ethical consideration was taken, and it was presented to the ethics review committee of Alneelain University, obstetrics, and gynecology department and approved, permission to conduct the study was requested from authorities of health care in Bashair Hospital, data was handled with a high degree of confidentiality throughout the study, and written informed Consent was taken from all participants in the study.
During the study period total of 4091 pregnant women came to the hospital, and 82 were diagnosed with ectopic pregnancy, so the incidence of ectopic pregnancies is found in approximately 2% of all pregnant women. Risk factors for ectopic pregnancy are strongly associated with conditions that cause alterations to the normal mechanism of fallopian tubal transport of fertilized ovum. Most study population age < 20 years 37.8% mean 2.1463, most women secondary school education 40.2%, multiparous were 53.7% and most gestational age of presentation (6-7) weeks 51.2% followed by (8-9) weeks 32.9% (Table 1).
| Table 1: Sociodemographic characteristic of the study population (n = 82) | ||||
| Chrematistic | Frequency | Percent | Mean | Std. Deviation |
| Age < 20 | 31 | 37.8 | 2.1463 | 1.19796 |
| 20-25 | 25 | 30.5 | ||
| 26-30 | 14 | 17.1 | ||
| 31-35 | 7 | 8.5 | ||
| > 35 | 5 | 6.1 | ||
| Education | ||||
| Primary | 28 | 34.1 | ||
| Secondary | 33 | 40.2 | 2.0122 | .94925 |
| University | 13 | 15.9 | ||
| Postgraduate | 8 | 9.8 | ||
| Parity | ||||
| Nulliparous | 23 | 28 | ||
| Multiparous | 44 | 53.7 | 1.9024 | .67786 |
| Grand multiparous | 15 | 18.3 | ||
| Gestational age | ||||
| 6-7 weeks | 42 | 51.2 | 1.9634 | .98689 |
| 8-9weeks | 27 | 32.9 | ||
| 10-11 weeks | 5 | 6.1 | ||
| ≥ 12weeks | 8 | 9.8 | ||
| Total | 82 | 100 | ||
In our study infection was the most identified risk for ectopic pregnancy at 29.3% followed by pelvic surgery at 15.9%, miscarriage at 13.4%, infertility at 12.2%, tubal surgery at 4.9%, tubal surgery is closely linked to the underlying tubal damage caused by a previous ectopic pregnancy or pelvic inflammatory disease. Previous ectopic pregnancy was 4.9%, IUCD was 3.6% and tubal ligation is the least risk factor at 2.4%, tubal ligation failures also confer a high risk for ectopic pregnancy (Table 2).
| Table 2: Risk of ectopic pregnancy among study population (n = 82). | ||||
| Risk | Frequency | Percent | Mean | Std |
| Infection | 24 | 29.3 | 1.7073 | .45779 |
| Surgery | 13 | 15.9 | 1.8415 | .36749 |
| Ectopic | 4 | 4.9 | 1.9512 | .21673 |
| Tubal surgery | 4 | 4.9 | 1.9512 | .21673 |
| Miscarriage | 11 | 13.4 | 1.8659 | .34291 |
| Infertility | 10 | 12.2 | 1.8780 | .32924 |
| IUCD | 3 | 3.6 | 1.9634 | .18890 |
| Tubal ligation | 2 | 2.4 | 1.9756 | .15521 |
| No risk | 11 | 13.4 | 1.8659 | .34291 |
| Total | 82 | 100 | ||
Twelve women who managed by surgery β-hCG not undertaken due to their emergency presentation and only one woman was in medical treatment (Table 3).
| Table 3: Management of ectopic pregnancy and β-hCG test (n = 82). | ||||
| β-hCG | Treatment | Total | ||
| Surgical | Medical | Medical and Surgical | ||
| Perfumed | 65 | 1 | 3 | 69 |
| Not performed | 12 | 1 | 0 | 13 |
| Total | 77 | 2 | 3 | 82 |
| Chi-square 2.317; p - value .02 | ||||
All women presented with shock were managed surgically, medical treatment in the form of methotrexate was offered to only two women who had no contraindications and three women of cervical ectopic pregnancy received misoprostol and surgical evacuation (Table 4).
| Table 4: Management of ectopic pregnancy and presentation symptoms (n = 82). | ||||
| Symptoms | Treatment | Total | ||
| Surgical | Medical | Medical and Surgical | ||
| Bleeding | 14 | 1 | 0 | 15 |
| Pain | 7 | 1 | 0 | 8 |
| Bleeding &pain | 37 | 0 | 2 | 39 |
| Shocked | 7 | 0 | 0 | 7 |
| Asymptomatic | 7 | 0 | 1 | 8 |
| Total | 77 | 2 | 3 | 82 |
| Chi-square 9.200; p - value .022 | ||||
All interstitial ectopic pregnancies 7, ampullary 47 women, and ovarian 6 and abdominal three women were managed by surgical methods. Two isthmic ectopics mangled with methotrexate and three cervical ectopics managed with medical and surgical evacuation p - value .000. Table 5. Regression risk factors for ectopic pregnancy (Table 6).
| Table 5: Management of ectopic pregnancy and site of ectopic pregnancy (n = 82). | ||||
| Site of ectopic | Treatment | Total | ||
| Surgical | Medical | Medical and Surgical | ||
| Interstitial | 7 | 0 | 0 | 7 |
| Isthmus | 5 | 2 | 0 | 7 |
| Ampullary | 47 | 0 | 0 | 47 |
| Fimbrial | 8 | 0 | 0 | 8 |
| Ovarian | 6 | 0 | 0 | 6 |
| Abdominal | 3 | 0 | 0 | 3 |
| Cervical | 1 | 0 | 3 | 4 |
| Total | 77 | 2 | 3 | 82 |
| Pearson Chi-Square 82.6; p - value .000 | ||||
| Table 6: Regression of risk factors of ectopic pregnancy (n = 82). | ||||
| Risk Factors | Had Risk | No risk | OR | 95% CI |
| Pelvic infections Yes 24 No 58 |
24 26 |
0 32 |
5.345 | 3.055-9.721 |
| Pelvic surgery Yes 13 No 69 |
11 39 |
2 30 |
4.231 | .871-20.541 |
| Miscarriage Yes 11 No 71 |
10 40 |
1 31 |
7.750 | .941-63.825 |
| Subfertility Yes 10 No 72 |
10 40 |
0 32 |
1.164, | 1.059-1.1279 |
| Previous ectopic Yes 4 No 78 |
4 46 |
0 32 |
1.060 | 1.001-1.122 |
| Tubal surgery Yes 4 N0 78 |
4 46 |
0 32 |
1.060 | 1.001-1.122 |
| Tubal ligation Yes 2 No 80 |
1 49 |
1 31 |
1.029 | .989-1.071 |
| IUCD Yes 3 No 79 |
2 1 |
48 31 |
1.044 | .994-1.096 |
Women managed by surgery received blood transfusion were 31 women while medically treated women do not need blood transfusion p - value .04 (Table 7).
| Table 7: Management of ectopic pregnancy and blood transfusion (n = 82). | ||||
| Symptoms | Treatment | Total | ||
| Surgical | Medical | Medical and Surgical | ||
| Transfused | 31 | 0 | 0 | 31 |
| Not transfused | 46 | 2 | 3 | 51 |
| Total | 77 | 2 | 3 | 82 |
| Pearson Chi-Square 3.237a; p - value .04 | ||||
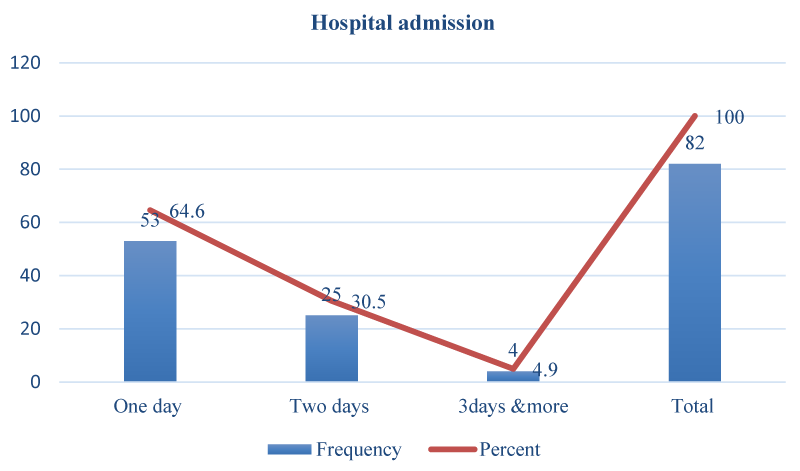
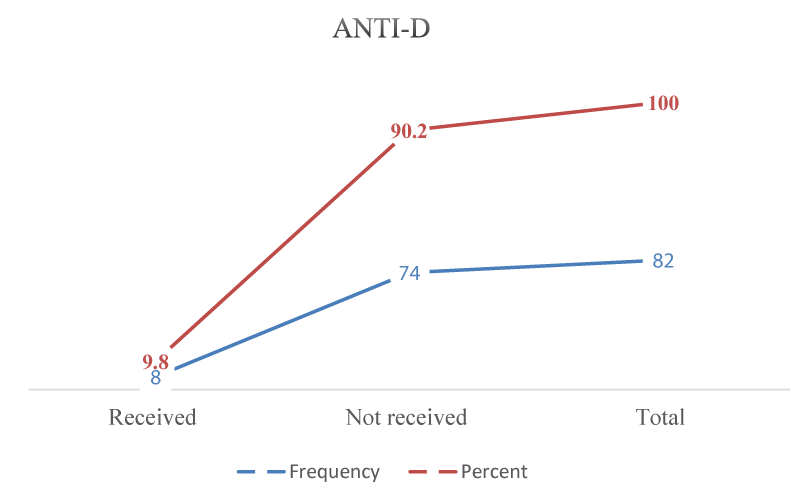
Outcomes of management of ectopic pregnancy with low hospital stay and no maternal mortality, those rhesus negative received Anti-D postoperative, and all women received antibiotics except two (Figures 1,2).
Figure 1: Hospital admission ectopic pregnancy and site of ectopic pregnancy (n = 82).
Figure 2: Anti-D received by ectopic pregnancy study populations (n = 82).
The incidence of ectopic pregnancy in our study was 2% similar to [2] and lower than [15] study conducted in Nigeria found an incidence of 2.2% comparable with [16]. Most women in this study are presented at less than the age of 26 years 68.3%, multiparous 53.7%, this is due to early marriage, neither age nor parity is significantly associated with the risk of ectopic pregnancy. Most cases of ectopic pregnancy were presented at 6-7 weeks 51.2%.
The common presentation of EP is vaginal bleeding and lower abdominal pain in a woman with amenorrhea was 47.5%, women who have an EP typically complain of brown vaginal discharge soon after a missed period, sometimes progressing to heavier bleeding similar to a miscarriage, only bleeding was 18.3%, abdominal pain 9.7%and asymptomatic women were 9.7%.
The nature, location, and severity of pain in ectopic pregnancy vary, it often begins as a colicky abdominal or pelvic pain that is localized to one side as the pregnancy distends the fallopian tube. The pain may become more generalized once the tube ruptures and hemoperitoneum develops and the patient may be presented with shock, shocked women were 8.5%. Other potential symptoms include presyncope, syncope, vomiting, diarrhea, shoulder pain, lower urinary tract symptoms, rectal pressure, or pain with defecation [17].
Diagnoses of ectopic pregnancy based on detection of Beta human chorionic gonadotropin β-hCG and ultrasound scan, β-hCG can be detected in pregnancy as early as eight days after ovulation [18]. All women underwent ultrasound scans and β-hCG was performed in 84.1% of women this was explained by the emergency presentation.
The most common identifiable risk factor among our patients was previous pelvic infections 29.3% OR 5.345 CI 3.055-9.721. Previous studies have reported a strong association between prior PID and EP with OR ranging from 2.0 to 10.1 [19].
Previous pelvic surgery was a major risk factor for developing ectopic pregnancy 15.9%, OR 4.231 95% CI (.871-20.541) which is comparable to [20,21], which it has been reported that previous tubal surgery is a major risk factor for EP with an estimated OR of 4.7 (2.4‑9.5).
Miscarriage was 13.4% among study women OR 7.750 95%CI(.941-63.825) similar to [20] and another study [22] and our study showed the association of prior spontaneous miscarriage with increased risk of EP because of this relationship most likely due to infection,
A strong association between a history of subfertility and risk of EP was also detected at 12.2%, OR 1.164, and 95% CI (1.059-1.1279) which may be due to a significant role of hyperstimulation in the induction of ovulation, with high estrogen levels [23]. This finding is further supported by another study [22], while previous ectopic pregnancy occurred in 4.9% of the cases OR 1.060 and 95% CI (1.001-1.122), tubal surgery 4.9%, OR 1.060 and 95% CI (1.001-1.122), previous tubal surgery it has been reported that previous tubal surgery is a major risk factor for EP with an estimated OR of 4.7 (2.4‑9.5) according to a meta‑analysis [21].
Tubal pregnancy may occur in a blocked tube with contralateral tubal patency in this case, the sperm migrates across the abdomen to fertilize an egg released from the blocked side, tubal ligation is the least risk factor 2.4%, OR 1.029 95% CI (.989-1.071) which is lower than [24] which could be explained by a small number of women with bilateral tubal ligation.
IUCD users had OR 1.044and 95% CI (.994-1.096), Early studies on risk factors of EP indicated that OR greater than one belonged to current IUD use [24,25], and in 13.4% of patients there were no identifiable risk factors.
In most of the patients, 65.8% reported abdominal pain as the main complaint at the time of presentation, which is in line with other studies vaginal bleeding was reported in 74.3% of the cases, making it the second most common reason for attending to the hospital. Similarly, vaginal bleeding was mentioned in many studies, whereas other studies found that pain and amenorrhea were the main symptoms which are comparable to [26]. Patients in hypovolemic shock accounted for 3.5%, which is lower than [26].
At the surgery of ectopic pregnancies, 57.3% were found in the ampullary part of the fallopian tube which is comparable to [15] which found the most common site of tubal ectopic was (ampullary in 52%, Fimbrial 9.7%, interstitial 8.5%, isthmus 8.5% and ovarian 7.3%), also our study similar to other studies [26-28].
The increased incidence of ovarian ectopic pregnancies is associated with the increased use of artificial reproductive technologies (ART) and intrauterine contraceptive devices (IUCDs) [29].
Presurgical diagnosis of cervical ectopic pregnancy was 4.8% and abdominal ectopic pregnancy was 3.6% which was undertaken by ultrasound scan.
All interstitial ectopic pregnancies 7, ampullary 47 women, ovarian 6, and abdominal three women were managed by surgical methods. Two isthmic ectopics mangled with methotrexate and three cervical ectopics managed with misoprostol and surgical evacuation p - value .000.
In this study two women with ectopic pregnancy were selected for treatment with methotrexate they had no contraindications, received a double dose regimen, and were offered follow-up, the time to resolution was 4 weeks, and the median time to resolution for ectopic pregnancies treated with methotrexate was 22 days, with the majority resolved within 5 weeks [30].
There were three women diagnosed with cervical ectopic pregnancy with minimal bleeding, they received medical treatment with misoprostol according to protocol and offered surgical evacuation, totally cured, and no postoperative complications.
All tubal ectopic pregnancies were managed by surgery offered salpingectomy, a radical surgery like salpingectomy could help avoid a recurrence of ectopic pregnancy at the same site. However, it is considered to decrease the chances of becoming pregnant. In a randomized control trial, the pregnancy rate among patients in the salpingostomy group was not better than that among those in the salpingectomy group when the contralateral tube was healthy [31,32], and all received antibiotics.
The blood transfusion rate was 37.8% and the probability of blood transfusion was also higher in ruptured ectopic pregnancy than in unruptured ectopic pregnancy. Thus, preoperative estimation of the amount of intra-abdominal blood loss using the ultrasound scan might be useful in predicting tubal ruptures which is like [22,33].
Hospital stays for one day among 64.6%, rhesus negative women 9.8% were received. Anti-D intramuscular. All products were sent for histopathology. No patient had uncontrolled bleeding and did not require a hysterectomy. No cornual ectopic pregnant patient could be managed medically because all were ruptured ectopic pregnancies. The success rate for surgical treatment was 100%, as shown in other studies [22,27].
A significant limitation of this study was the small number of study populations, as well as the study period and laparoscopic surgery not used in the management of ectopic pregnancy. The study was conducted at one hospital, multicenter study can help in comparison. However, the study’s main strength was that the study covered all patients, and several types of ectopic pregnancy in a low-income setting and showed patterns that could be reviewed for future clinical and research uptake.
This study concluded that ectopic pregnancy is a common and serious problem, many patients have no documented risk factors and no physical indications of ectopic pregnancy. Our study demonstrated major risk factor for ectopic pregnancy was an infection which could be reduced by the detection of genital tract infection and treated prepregancy at the genitourinary tract clinic. Early diagnosis of ectopic pregnancy will reduce morbidity and mortality.
Most women present with ruptured ectopic pregnancy so public health awareness should be raised and doctors should be trained for ultrasound scans to enable early detection of ectopic pregnancy. Surgical management of ectopic pregnancy is a lifesaving intervention. Medical treatment has a high success rate in selected cases who has no contraindications. Although different management options are available, the best outcome is achieved if the management of EP is done at the earliest without any delay. Further study to evaluate medical, laparoscopic management and auditing of diagnosis.
Recommendations
To the hospital management, an excellent quality ultrasonography machine should be available, and 24-hour ultra-sonographer radiologists should be present to train the medical team. The provision of facilities and training of healthcare professionals on the modern management of ectopic pregnancy will lead to improved treatment outcomes.
I acknowledge my sincere thanks to Prof. Mandor Mohamed Ibrahim for participation and all my patients who have given their consent for participation in the study.
Ethical consideration
Ethical considerations taken from Alneelain University faculty of medicine, head department of obstetrics and gynecology and Bahair Teaching Hospital.
- American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 193: Tubal Ectopic Pregnancy. Obstet Gynecol. 2018 Mar;131(3):e91-e103. doi: 10.1097/AOG.0000000000002560. Erratum in: Obstet Gynecol. 2019 May;133(5):1059. PMID: 29470343.
- Hendriks E, Rosenberg R, Prine L. Ectopic Pregnancy: Diagnosis and Management. Am Fam Physician. 2020 May 15;101(10):599-606. PMID: 32412215.
- Elson CJ, Salim R, Potdar N, Chetty M, Ross JA, Kirk EJ. On behalf of the Royal College of Obstetricians and Gynecologists. Diagnosis and management of ectopic pregnancy. BJOG 2016; 123: e15-e55.
- Mullany K, Minneci M, Monjazeb R, C Coiado O. Overview of ectopic pregnancy diagnosis, management, and innovation. Womens Health (Lond). 2023 Jan-Dec;19:17455057231160349. doi: 10.1177/17455057231160349. PMID: 36999281; PMCID: PMC10071153.
- Stabile G, Mangino FP, Romano F, Zinicola G, Ricci G. Ectopic Cervical Pregnancy: Treatment Route. Medicina (Kaunas). 2020 Jun 12;56(6):293. doi: 10.3390/medicina56060293. PMID: 32545627; PMCID: PMC7353881.
- Dvash S, Cuckle H, Smorgick N, Vaknin Z, Padoa A, Maymon R. Increase rate of ruptured tubal ectopic pregnancy during the COVID-19 pandemic. Eur J Obstet Gynecol Reprod Biol. 2021 Apr;259:95-99. doi: 10.1016/j.ejogrb.2021.01.054. Epub 2021 Jan 29. PMID: 33636621; PMCID: PMC7968738.
- Barash JH, Buchanan EM, Hillson C. Diagnosis and management of ectopic pregnancy. Am Fam Physician. 2014 Jul 1;90(1):34-40. PMID: 25077500.
- Mathlouthi N, Slimani O, Ferchichi A, Ben Temime R, Makhlouf T, Attia L, Chachia A. Traitement médical de la grossesse extra-utérine [Medical treatment of ectopic pregnancy]. Tunis Med. 2013 Jul;91(7):435-9. French. PMID: 24008873.
- Houser M, Kandalaft N, Khati NJ. Ectopic pregnancy: a resident's guide to imaging findings and diagnostic pitfalls. Emerg Radiol. 2022 Feb;29(1):161-172. doi: 10.1007/s10140-021-01974-7. Epub 2021 Oct 7. PMID: 34618256.
- Cheng LY, Lin PY, Huang FJ, Kung FT, Chiang HJ, Lin YJ, Lan KC. Ectopic pregnancy following in vitro fertilization with embryo transfer: A single-center experience during 15 years. Taiwan J Obstet Gynecol. 2015 Oct;54(5):541-5. doi: 10.1016/j.tjog.2015.08.004. PMID: 26522107.
- Alur-Gupta S, Cooney LG, Senapati S, Sammel MD, Barnhart KT. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: a meta-analysis. Am J Obstet Gynecol. 2019 Aug;221(2):95-108.e2. doi: 10.1016/j.ajog.2019.01.002. Epub 2019 Jan 7. PMID: 30629908; PMCID: PMC6612469.
- Brady PC. New Evidence to Guide Ectopic Pregnancy Diagnosis and Management. Obstet Gynecol Surv. 2017 Oct;72(10):618-625. doi: 10.1097/OGX.0000000000000492. PMID: 29059454.
- ACOG practice bulletin no. 193: Tubal ectopic pregnancy [published correction appears in Obstet Gynecol. 2019; 133(5):1059]. Obstet Gynecol. 2018; 131(3): 91-103.
- Elito Júnior J, Araujo Júnior E. Medical Treatment for Ectopic Pregnancy during the COVID-19 Pandemic. Rev Bras Ginecol Obstet. 2020 Dec;42(12):849-850. doi: 10.1055/s-0040-1718438. Epub 2020 Dec 21. PMID: 33348404; PMCID: PMC10309203.
- Nzaumvila DK, Govender I, Ogunbanjo GA. An audit of the management of ectopic pregnancies in a district hospital, Gauteng, South Africa. Afr J Prim Health Care Fam Med. 2018 Oct 30;10(1):e1-e8. doi: 10.4102/phcfm.v10i1.1757. PMID: 30456972; PMCID: PMC6244319.
- Clement WFC, Ijeoma NEE, Ledee KP. Ectopic pregnancy in Rivers State University Teaching Hospital, Port Harcourt, southern Nigeria: a five- year review. World Journal of Advanced Research and Reviews. 2020; 6: 126.
- Newbatt E, Beckles Z, Ullman R, Lumsden MA; Guideline Development Group. Ectopic pregnancy and miscarriage: summary of NICE guidance. BMJ. 2012 Dec 12;345:e8136. doi: 10.1136/bmj.e8136. PMID: 23236034.
- Barnhart KT, Guo W, Cary MS, Morse CB, Chung K, Takacs P, Senapati S, Sammel MD. Differences in Serum Human Chorionic Gonadotropin Rise in Early Pregnancy by Race and Value at Presentation. Obstet Gynecol. 2016 Sep;128(3):504-511. doi: 10.1097/AOG.0000000000001568. PMID: 27500326; PMCID: PMC4993627.
- Barnhart KT, Sammel MD, Gracia CR, Chittams J, Hummel AC, Shaunik A. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006 Jul;86(1):36-43. doi: 10.1016/j.fertnstert.2005.12.023. Epub 2006 May 30. PMID: 16730724.
- Moini A, Hosseini R, Jahangiri N, Shiva M, Akhoond MR. Risk factors for ectopic pregnancy: A case-control study. J Res Med Sci. 2014 Sep;19(9):844-9. PMID: 25535498; PMCID: PMC4268192.
- Ankum WM, Mol BW, Van der Veen F, Bossuyt PM. Risk factors for ectopic pregnancy: a meta-analysis. Fertil Steril. 1996 Jun;65(6):1093-9. PMID: 8641479.
- Berek JS. Berek and Novaks Gynecology. 14th ed. Philadelphia: Lippincott Williams and Wilkins; 2007.
- Bouyer J, Rachou E, Germain E, Fernandez H, Coste J, Pouly JL, Job-Spira N. Risk factors for extrauterine pregnancy in women using an intrauterine device. Fertil Steril. 2000 Nov;74(5):899-908. doi: 10.1016/s0015-0282(00)01605-8. PMID: 11056230.
- Farquhar CM. Ectopic pregnancy. Lancet. 2005 Aug 13-19;366(9485):583-91. doi: 10.1016/S0140-6736(05)67103-6. PMID: 16099295.
- Bouyer J, Coste J, Shojaei T, Pouly JL, Fernandez H, Gerbaud L, Job-Spira N. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003 Feb 1;157(3):185-94. doi: 10.1093/aje/kwf190. PMID: 12543617.
- Marchbanks PA, Annegers JF, Coulam CB, Strathy JH, Kurland LT. Risk factors for ectopic pregnancy. A population-based study. JAMA. 1988 Mar 25;259(12):1823-7. PMID: 3343790.
- Verma ML, Singh U, Solanki V, Sachan R, Sankhwar PL. Spectrum of Ectopic Pregnancies at a Tertiary Care Center of Northern India: A Retrospective Cross-sectional Study. Gynecol Minim Invasive Ther. 2022 Feb 14;11(1):36-40. doi: 10.4103/GMIT.GMIT_1_21. PMID: 35310127; PMCID: PMC8926047.
- Igwegbe A, Eleje G, Okpala B. An appraisal of the management of ectopic pregnancy in a nigerian tertiary hospital. Ann Med Health Sci Res. 2013 Apr;3(2):166-70. doi: 10.4103/2141-9248.113655. PMID: 23919183; PMCID: PMC3728856.
- Panti A, Ikechukwu NE, lukman OO, Yakubu A, Egondu SC, Tanko BA. Ectopic pregnancy Usmanu Danfodiyo University Teaching Hospital Sokoto: A ten-year review. Ann Nigerian Med. 2012; 6(2):87-91.
- Tabassum M, Atmuri K. The Unexpected Ovarian Pregnancy at Laparoscopy: A Review of Management. Case Rep Obstet Gynecol. 2017;2017:9856802. doi: 10.1155/2017/9856802. Epub 2017 Sep 11. PMID: 29085687; PMCID: PMC5612308.
- Davenport MJ, Lindquist A, Brownfoot F, Pritchard N, Tong S, Hastie R. Time to resolution of tubal ectopic pregnancy following methotrexate treatment: A retrospective cohort study. PLoS One. 2022 May 24;17(5):e0268741. doi: 10.1371/journal.pone.0268741. PMID: 35609041; PMCID: PMC9129037.
- Mol F, van Mello NM, Strandell A, Strandell K, Jurkovic D, Ross J, Barnhart KT, Yalcinkaya TM, Verhoeve HR, Graziosi GCM, Koks CAM, Klinte I, Hogström L, Janssen ICAH, Kragt H, Hoek A, Trimbos-Kemper TCM, Broekmans FJM, Willemsen WNP, Ankum WM, Mol BW, van Wely M, van der Veen F, Hajenius PJ; European Surgery in Ectopic Pregnancy (ESEP) study group. Salpingotomy versus salpingectomy in women with tubal pregnancy (ESEP study): an open-label, multicentre, randomised controlled trial. Lancet. 2014 Apr 26;383(9927):1483-1489. doi: 10.1016/S0140-6736(14)60123-9. Epub 2014 Feb 3. PMID: 24499812.
- Li PC, Lin WY, Ding DC. Risk factors and clinical characteristics associated with a ruptured ectopic pregnancy: A 19-year retrospective observational study. Medicine (Baltimore). 2022 Jun 17;101(24):e29514. doi: 10.1097/MD.0000000000029514. PMID: 35713461; PMCID: PMC9276220.