More Information
Submitted: July 31, 2023 | Approved: August 09, 2023 | Published: August 10, 2023
How to cite this article: Otoikhila OC, Seriki SA. A Comparative Study of Serum Sodium and Potassium Levels across the Three Trimesters of Pregnancy. Clin J Obstet Gynecol. 2023; 6: 108-116.
DOI: 10.29328/journal.cjog.1001137
Copyright License: © 2023 Otoikhila OC, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Serum potassium; Serum sodium; Pregnancy hormones; Blood pressure; Trimesters
A Comparative Study of Serum Sodium and Potassium Levels across the Three Trimesters of Pregnancy
Otoikhila OC and Seriki SA*
Department of Human Physiology, College of Medical Sciences, Edo State University, Uzairue, Nigeria
*Address for Correspondence: Samuel Adinoyi Seriki, Department of Human Physiology, College of Medical Sciences, Edo State University, Uzairue, Nigeria, Email: [email protected]; [email protected]
Aim: To evaluate the serum sodium and potassium levels in the three trimesters of pregnancy in women.
Methods: Four groups of healthy women between the ages of 20 and 30 years, volunteered for this study. Group 1: Non-pregnant women (control), Group 2: Pregnant women in their first trimester, Group 3: Pregnant women in their second trimester, Group 4: Pregnant women in their third trimester. Blood samples were collected by standard aseptic method and serum samples were analyzed for serum levels of sodium and potassium by the ion selective electrode method. Results were presented as MEAN ± SEM in tables and figures, p < 0.05 was regarded as statistically significant.
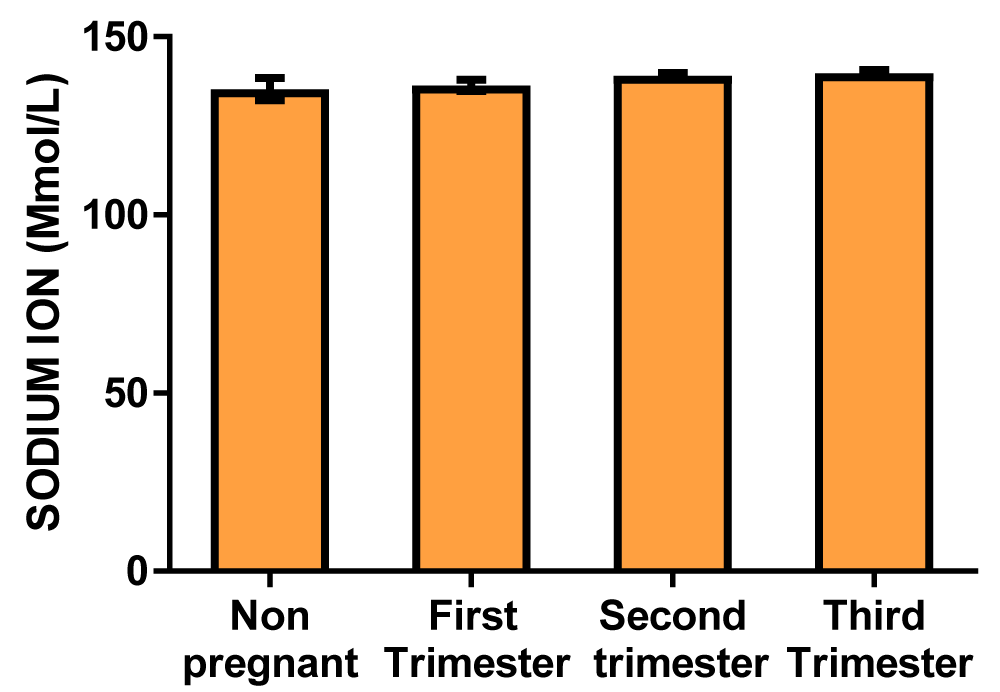
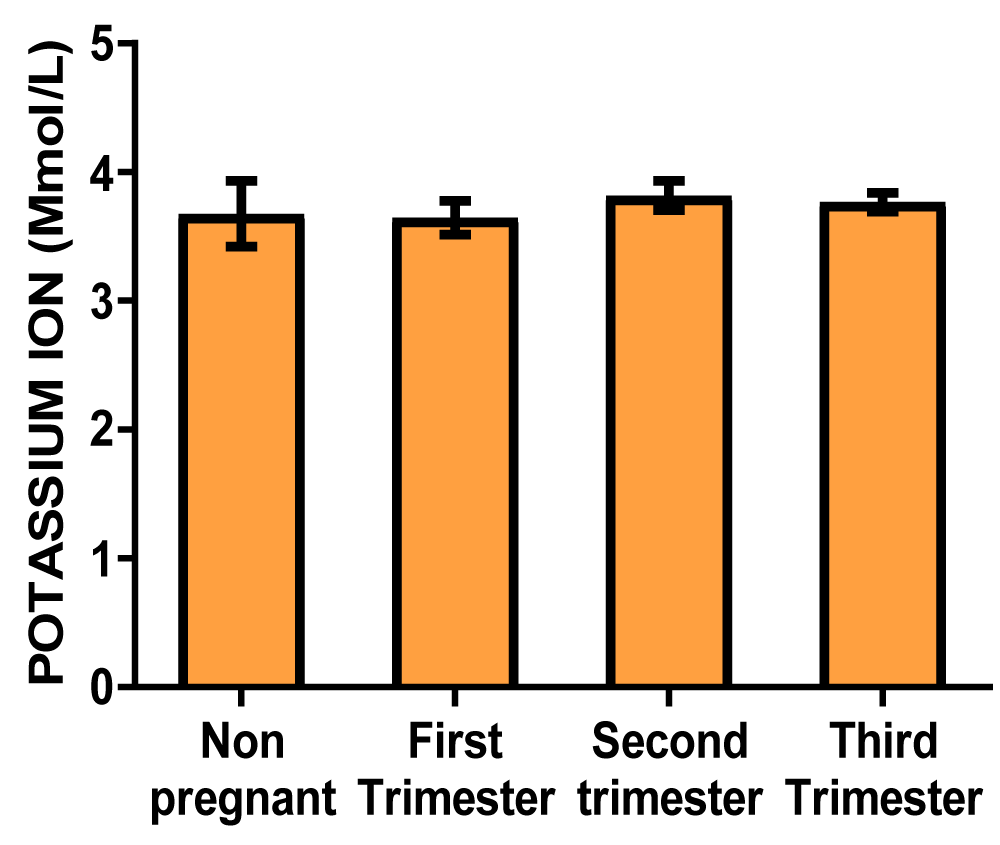
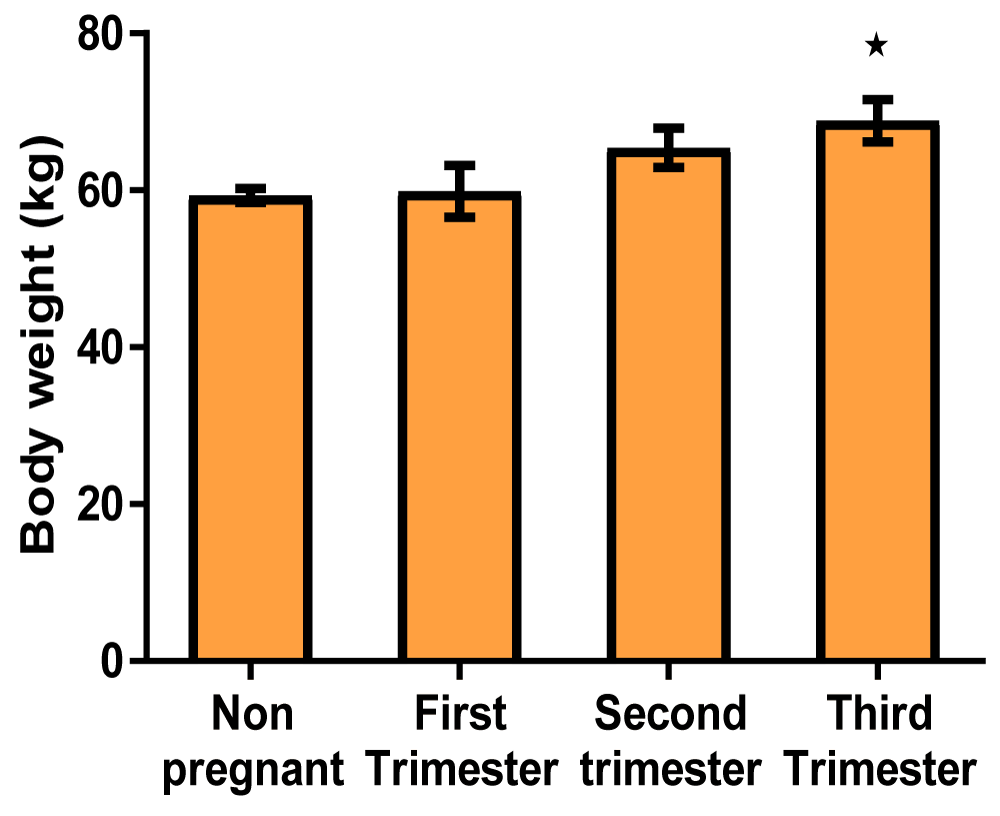
Results: The serum sodium concentrations in mmol/L were 135.3 ± 3.09, 136.3 ± 1.55, 139.0 ± 0.78, 139.8 ± 0.91 for control, first-trimester, second-trimester, and third-trimester subjects respectively. The potassium concentrations in mmol/L were 3.678 ± 0.26, 3.687 ± 0.13, 3.820 ± 0.11, 3.767 ± 0.07 for control, first-trimester, second trimester and third-trimester subjects respectively. The Bodyweight values in kg were 72.13 ± 2.11, 74.73 ± 2.05, 75.00 ± 1.72, 81.56 ± 4.24 for control, first-trimest, second-trimester, and third-trimester subjects respectively.
Conclusion: Results indicate that the hormones of pregnancy altered the body weight of pregnant women, but did not change the serum sodium and potassium level across the three trimesters of pregnancy in women. This is an indication that the kidneys of healthy pregnant women can handle serum electrolyte load during the period of pregnancy.
Pregnancy is also called gravidity and gestation. It is the period during which one or more babies develop inside the uterus of a woman. The period of gestation in humans is about 40 weeks: it is calculated from the last menstrual period and ends with childbirth. But when it is measured from conception (the time a sperm fertilizes an egg in the fallopian tube) it comes down to about 38 weeks (266 days) [1]. Pregnancy usually occurs naturally through sexual intercourse or by assisted technology called artificial insemination. The period of pregnancy is divided into three stages which are called trimesters, with each trimester having its own specific developmental process [2].
The most important organ in maintaining appropriate fluid and electrolyte balance is the kidney. Other factors such as physiological stress and hormonal changes especially during pregnancy play a role [3]. Normal pregnancy leads to physiological and anatomical changes in the renal system that is caused by the gravid uterus, and increased concentration of progesterone and estrogen. These changes can have attendant effects on plasma volume. For instance, the rise in the secretion of estrogen lowers the operating point for osmoregulation of arginine vasopressin and thirst, leading to an increase in plasma volume. In addition, renal functional capacity is enhanced by marked vasodilatation, an increase in renal blood flow with an overall increase in glomerular filtration rate, with the greatest GFR recording in the 2nd trimester. However, it should be noted that the increase in plasma volume is not connected with an increase in the number of nephrons; rather it is the direct outcome of the increase in renal vascular and interstitial volume [4].
There are conflicting reports about the serum concentration of the chief electrolytes: potassium (K+) and sodium (Na+) during the three trimesters of pregnancy. Previous research found a decrease in K+ and Na+ concentrations from 10 – 20 weeks, in multiparous but not in primigravidae pregnancies. More results from both pregnancies showed a significant increase in K+ concentration between 28 – 37 weeks, with no change in Na+. Reports from other authors did not find any change in electrolyte values between the control and the three trimesters of pregnancy [5,6]. These references are properly placed. The paragraph noted that while some articles observed some changes in electrolyte concentrations through the trimesters of pregnancy, the articles referenced did not notice any change. That’s exactly the point intended in the paragraph.
However, the natriuretic and kaliuretic influence of pregnancy may alter the electrolyte concentrations or balance as pregnancy advances through the three trimesters. This disparity in electrolyte concentration may be a result of the metabolic effects of the pregnancy hormones such as progesterone, estrogen, and relaxin across the trimesters of pregnancy. Thus, the results of biochemical tests on serum electrolytes during pregnancy may differ from the normal reference ranges in the literature.
Pregnancy
Pregnancy is the physiologic process of a developing fetus within the maternal body. Pregnancy occurs both by sexual intercourse and through assisted reproductive technology procedures [7]. Oftentimes, pregnancy ends in a live birth, but other times it may end in spontaneous miscarriage, induced abortion, or a stillbirth. Term pregnancy describes a pregnancy that stays for a period of 37 to 39 weeks. Neonates born in less than 37 completed weeks of pregnancy are classified as preterm, whereas those delivered beyond 42 weeks are classified as post-term [8].
Pregnancy begins with conception, which is the fertilization of an egg by a sperm to form a zygote and continues through to the birth of the individual. The period of gestation varies among animals. Human gestation is 266 days; 0 to 13th week (first trimester); 14th to 26th week (second trimester); 27th to 40th week (third trimester) [9].
The three trimesters of pregnancy have different emotional and physical happenings that make them unique for the mother and her baby. In addition, each trimester comprises unique and specific fetal development [2].
Towards the end of the third trimester, there may be discomfort as the fetus moves into position in the woman's lower abdomen. Edema (swelling of the ankles), back pain, and balance problems are sometimes experienced by the mother during this period. The fetus continues to increase rapidly in weight, size, and physical development.
Physiological adaptation of the renal system to pregnancy
It has already been established that pregnancy is a state of volume expansion and vasodilation, chiefly stimulated by progesterone and estrogen, in association with the careful coordination of several hormones. The kidneys are the main organs concerned with the regulation of body fluid, including blood volume. Pregnancy affects the anatomical, biochemical, and physiological aspects of the renal system. These changes occur from as early as 6 weeks after conception. Anatomical changes such as an increase in the size of the kidneys, and dilatation of the urinary collecting system involving calices, renal pelvis, and ureters have long been observed [10]. Renal and systemic hemodynamics is characterized by vasodilation and volume expansion, following alteration of the renal vascular and tubular responsiveness to the circulating hormones [11]. The Physiological impact of pregnancy on the kidney is widespread, involving all aspects of kidney function, including but not limited to hemodynamics, glomerular filtration, and tubular handling of water, electrolytes, and other substances and alterations of the renin-angiotensin system (RAS) [10].
Renal handling of sodium and potassium during pregnancy
Despite the filtered load of sodium increasing during pregnancy, reabsorption from renal tubules is also increased. Such that there is a net retention of 900 mEq – 950 mEq of sodium during pregnancy, which helps to sustain the plasma volume increase in the dilated systemic vasculature. Although the mechanisms of sodium retention are unclear, it has been suggested that it may involve a balance between natriuretic factors such as increased GFR, decreased renal vascular resistance, decreased oncotic pressure, and decreased serum albumin, vasodilatory prostaglandins, progesterone, increased atrial natriuretic peptide and anti-natriuretic factors such as increased renin, aldosterone, human placental lactogen, estrogen and corticosterone. Relaxin also mediates sodium balance. However, pregnant women remain in homeostatic balance, as the retention of sodium is compensated by the water accumulation [12].
Potassium excretion is constant throughout pregnancy, with an increase in tubular reabsorption adapting to alterations in filtered load. This leads to retention and an increase in total body potassium stores by approximately 300 mEq – 350 mEq by the end of gestation. However, the maternal serum potassium level does not rise as the additional potassium is used by the maternal and fetal tissues. The retention of potassium occurs due to the anti-kaliuretic effects of progesterone that antagonize the kaliuretic effects of aldosterone [12].
Electrolytes
Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and conducting action potentials in the nerves and muscles. Sodium, potassium, and chloride are significant electrolytes along with magnesium, calcium, phosphate, and bicarbonates. Electrolytes come from our food and fluids. “Electrolyte” is the umbrella term for particles that carry a positive or negative electric charge [13]. An electrolyte is defined as a chemical substance that when dissolved in water or melted, dissociates into electrically charged particles called ions. Thus, electrolytes are capable of conducting an electric current [13]. Positively and negatively charged molecules called ions, are found within cells, between cells, in the bloodstream, and in other fluids throughout the body. The primary ions of electrolytes are sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), chloride (Cl−), hydrogen phosphate (HPO42−), and hydrogen carbonate (HCO3−). The electric charge symbols of plus (+) and minus (−) indicate that the substance is ionic in nature and has an imbalanced distribution of electrons, the result of chemical dissociation [14]. Thus, the electrolytes with positive charge include; sodium, potassium, calcium, and magnesium. The negative ions are chloride, bicarbonate, and phosphate. Common electrolytes that are measured in blood testing include sodium, potassium, chloride, and bicarbonate [15].
Electrolytes can also be described as minerals that carry an electric charge and are located in extracellular and intracellular fluids. Within the extracellular fluid (ECF), the major cation is sodium and the major anion is chloride. The major cation in the intracellular fluid (ICF) is potassium. Electrolytes are found in sweat and urine and are vital to specific processes that keep the body functioning as it should. When these minerals dissolve in a fluid, they form positive or negative ions used in metabolic processes [16].
Sodium
Sodium is the major electrolyte in the extracellular fluid (ECF) with about 98% of its totality being in the ECF and only 2% being in the intracellular fluid (ICF) with a reference range of 135 mEq/L - 145 mEq/L. The human body contains 105 grams of sodium located mainly in the bones, extracellular fluids (like the serum), and tissues. Bone crystals play a reservoir role and release sodium in case of serum level deficiency [17].
Sodium disorders
Serum sodium levels below 135 mEq/L result in a condition termed hyponatremia caused by low levels of sodium or excess water in relation to the amount of sodium. This condition is common in postoperative patients [18]. A drop in sodium concentration causes cellular edema which affects the central nervous system and leads to depression and cerebral edema [19]. Sodium levels above 145 mEq/L result in hypernatremia, a condition that is generally associated with a hyperosmolar state where a fluid volume exists. The increase in extracellular sodium causes intracellular fluid to shift out of the cells into extracellular spaces, which results in cellular dehydration [20].
Hyponatremia is associated with such symptoms as confusion, lethargy, fatigue, headache, nausea, vomiting, loss of appetite, spasms, cramps, seizures, depressed neural reflexes, and ST elevation on the electrocardiogram (ECG). If the serum sodium concentration falls further; stupor, neuromuscular hyperexcitability, hyperreflexia, seizures, and coma appear, which may lead to death [21].
The major symptoms of hypernatremia are connected with brain cell shrinkage. Depending on the duration of the ions' abnormal level and on actual blood volume, the signs of hypernatremia may include thirst, neuromuscular excitability, confusion, seizures, coma, and cardiopulmonary arrest [22]. Sodium is essential in the human body. It plays a vital role in maintaining the concentration and volume of the extracellular fluid and accounts for most of the osmotic activity of plasma. Serum sodium levels are maintained by feedback loops involving the kidneys, adrenal glands, and hypothalamus [2].
Serum sodium in pregnancy
Normal serum sodium ion concentration for pregnant women is 129 mMoL/L - 149 mMoL/L. The total amount of sodium in a woman weighing 60 kg is 53g. Out of this 20g is in bone and is virtually immobile, taking little or no part in the daily exchanges of sodium ions throughout the body. The fatty tissue of the body contains almost no sodium ions and each 100g of fat-free body weight contains 0.109g of sodium [23].
Pregnant women are susceptible to sodium imbalances due to pregnancy-related changes in hormones and resultant maternal physiological adaptations that affect the metabolism, cardiovascular system, and urinary system of the expectant mother. In addition, the use of medicines that affect electrolyte excretion or retention like diuretics, also commonly causes sodium imbalance [24].
The role of aldosterone in regulating sodium
Aldosterone is secreted from the adrenal cortex as the final step in the renin-angiotensin-aldosterone pathway. In response to reduced pressure in the afferent arterioles of the kidneys (such as seen in hypotension), the juxtaglomerular cells produce renin. Renin leads to increased angiotensin II, which causes both vasoconstriction and the release of aldosterone. Aldosterone acts in the renal tubules to increase the reabsorption of both sodium and water. In essence, aldosterone causes the body to retain more salt water (saline), thereby increasing extracellular volume without altering sodium concentration [25].
Potassium
Potassium (K+) is the major element in the intracellular fluid with about 98% of its totality in the ICF, leaving 2% in the ECF with a reference range of 3.5 mEq/L - 5.0 mEq/L. Thus, serum potassium is a small fraction of whole-body potassium. Potassium is one of the most important ionic components of the intracellular fluid since the majority of its concentration is in the ICF. Potassium is a necessary element playing a physiological role in multiple processes such as the electrical impulse conduction and the contraction of smooth and skeletal muscles, including the heart. For example, it is the increased efflux of K+ ions from cardiomyocytes that determines their return to the resting state. Potassium also facilitates cell membrane function and proper enzyme activity. Its role is especially significant in excitable cells, such as neurons. The resting potential of these cells depends mainly on potassium since their membrane is the most permeable to this ion [26].
Regulation of potassium
Potassium homeostasis is highly influenced by the activities of the sodium and potassium pump (Na+-K+-ATPase) which facilitates the active transport of sodium and potassium ions across the cell membrane against their concentration gradients. The Na+-K+-ATPase is found in the membrane of almost all animal cells and it pumps sodium ions out of the cell and potassium ions into the cell. This pump keeps the K+ balance between the ICF and ECF primarily through a buffering that involves hydrolysis of ATP to generate energy, and for each ATP hydrolyzed, two K+ are transported inside while three Na+ are pushed out of the cell. This maintains high Na+ in the ECF and high K+ in the ICF and creates an electrical gradient [27]. The cytoplasm becomes negatively charged as the number of Na+ leaving the cell is higher than the number of K+ entering the cell. This electrical gradient is used in neurons and muscles for nervous system function and muscular contraction [28]. This K+ and Na+ interchange influences K+ homeostasis. When the body needs to retain more Na+, renal K+ secretion is induced leading to an increase in the delivery and reabsorption of Na+ by the distal nephron. This conversely forces the passive K+ efflux across the apical membrane [29].
The kidney moderates the activities of aldosterone which when overproduced facilitates K+ loss. Under normal conditions, intracellular K+ buffers the effect of a fall in extracellular K+ concentrations by moving into the extracellular space but the overproduction of aldosterone affects this balance and causes continued loss of K+ [30]
There are also genes involved in K+ homeostasis, especially WNK genes which act at the distal convoluted tubule (DCT). The WNK genes act as a molecular switch and activate the thiazide-sensitive NaCl cotransporter (NCC). Low intracellular chloride level triggers the activation of NCC by WNK kinases resulting in the reabsorption of potassium at the DCT and preventing the loss of potassium [31]. Mutations of the WNK1 and WNK4 genes were observed in some patients with hyperkalemia and hypertension caused by pseudohypoaldosteronism type II [32].
Another organ involved in K+ homeostasis is the colon. The colon is the major site of gut regulation of potassium excretion. Potassium excretion via the colon is minimal during normal conditions but its role increases as the renal function worsens as seen in renal insufficiency or during acute potassium overload when the kidneys are overwhelmed [33].
Skeletal muscle is also implicated in extracellular K+ concentration regulation. According to a study on rats using a K+ clamp technique, there was a decrease in muscle sodium pump pool size due to K+ deprivation. Also, glucocorticoid treatment induced an increase in muscle Na+-K+-ATPase alpha2 levels. Furthermore, the body can adapt to changes in renal and extrarenal K+ balance without significantly altering plasma K+ level [34]. Although the Na+-K+-ATPase mechanism is still under investigation, there is evidence that insulin may influence the activity and expression of muscle Na+-K+-ATPase. It has been reported that insulin forces K+ into the cells. Insulin apart from regulating glucose metabolism after a meal also shifts dietary K+ into cells until the kidney excretes the K+ load to re-establish K+ homeostasis [34].
Potassium disorders
Conditions, where serum potassium levels are below 3.5 mEq/L is termed hypokalaemia and can be found in 20% of hospitalized patients. Potassium may also be lost through kidney excretion in association with metabolic alkalosis and hyperaldosteronism. Potassium levels below 3.0 mEq/L can cause a problem with cardiovascular and neuromuscular function causing compromised respiratory function. Hypokalaemia also results in glucose intolerance by depressing insulin release from the pancreas [35]. Cardiac and/or respiratory arrest can also result from very low levels of potassium [36]. Hyperkalaemia is a condition where the serum potassium level is above 5.0 mEq/L and is most often related to kidney failure due to inadequate kidney function. Hyperkalaemia could lead to fatal arrhythmias if not diagnosed and managed early enough [36].
Preeclampsia and pregnancy-induced hypertension
Preeclampsia is a disorder that clinically manifests as the triad of hypertension, and proteinuria, and occurs with or without edema after 20 weeks gestation in previously normotensive women. Preeclampsia a pregnancy-specific syndrome is one of the most common causes of Maternal and fetal morbidity and mortality. It is specific to human pregnancy [37]. Although studies have reported that serum calcium and magnesium levels have a vasomotor activity on blood vessels in pregnancy, whilst others have reported a varying conclusion on the effects of serum sodium and potassium levels on vasomotor activity during pregnancy. In all of this, serum sodium and potassium levels have been observed to be reduced in both preeclampsia and pregnancy-induced hypertension as compared to normotensive pregnant and non-pregnant women [38].
The etiology of preeclampsia is yet to be fully understood in spite of numerous studies that have been conducted. What is known is that the syndrome is accompanied by a disturbance in electrolyte balance in pregnant females because of substantial alterations in intracellular water concentration. This is related to changes in the cell membrane, which appears to be responsible for various pathological changes in preeclampsia. The suggested mechanism of action states that these alterations involve changes in the transport of sodium ions both at systemic and intracellular levels [39]. Going further, an excessive intake of sodium chloride leads to sodium and water retention, the expansion of extracellular fluid and intravascular volume, increased venous return, and an elevated cardiac index. As elevated blood flow to the tissue continues, whole-body autoregulation takes place, with a subsequent increase in the total peripheral resistance and the eventual development of hypertension [40]. The peripheral arterial vasodilation hypothesis of sodium and water retention in pregnancy and its implications for the pathogenesis of preeclampsia-eclampsia explain that with increased endothelial damage, sodium retention and increased sensitivity to angiotensin lead to hypertension, edema, and proteinuria, the diagnostic triad of preeclampsia-eclampsia [38].
Laboratory values of serum sodium and potassium [41].
Serum Sodium:
Normal Range: 135 mmol/L to 145 mmol/L
Mild-moderate Hyponatremia: 125 mmol/L to 135 mmol/L, Severe: less than 125 mmol/L
Hypernatremia: Mild-moderate: 145 mmol/L to 160 mmol/L, Severe: over 160 mmol/L
Serum Potassium:
Normal Range: 3.6 mmol/L to 5.5 mmol/L
Hypokalemia: Mild Hypokalemia under 3.6 mmol/L, Moderate: 2.5 mmol/L, Severe: greater than 2.5 mmol/L
Hyperkalemia: Mild hyperkalemia: 5 mmol/L to 5.5 mmol/L, Moderate- 5.5 to 6.5, Severe: 6.5 mmol/L to 7 mmol/L.
Purpose of study
The purpose of the study is to evaluate the serum sodium and potassium levels across the three trimesters of pregnancy in women and to compare them with non-pregnant women to determine if pregnancy tasks the kidneys beyond capacity during pregnancy.
Subjects
A total of 80 women between the ages of 20 and 30 years, attending the clinic in the Edo State University Teaching Hospital, Auchi, were used as volunteers for the study. Sixty of the women were pregnant women and divided into three groups of first, second, and third trimesters with 20 subjects in each group. The control subjects were non-pregnant women.
Thus, the selected persons were divided into four groups for analysis as follows:
Group 1 - The control group - 20 non-pregnant women.
Group 2 – Study group - 20 pregnant women, in the first trimester of pregnancy.
Group 3 – Study group - 20 pregnant women, in the second trimester of pregnancy.
Group 4 – Study group - 20 pregnant women, in the third trimester of pregnancy.
Exclusion criteria: Diabetic, hypertensive, and anemic subjects were excluded from the study. Others that were excluded are subjects with vomiting, diarrhea, chronic alcohol consumption, renal disease, and chronic illnesses.
Inclusion criteria: This study included booked patients with booking laboratory parameters that were within normal range.
Blood sample collection and analysis
Three (3) ml of venous blood was collected under the standard aseptic method from the antecubital fossa without the application of a tourniquet to prevent disrupting the hemorheological properties of the blood into a lithium heparin sample bottle. The blood was allowed to clot and after centrifugation serum was collected. The blood samples were processed within 4-6 hours. The serum samples were analyzed for serum levels of sodium and potassium by the ion-selective electrode method [42]. The values across the three trimesters were compared with laboratory reference values of serum sodium and potassium electrolytes.
The normal values of serum electrolytes in women are:
• Serum Sodium – 135 to 145 meq/L
• Serum Potassium – 3.5 to 5 meq/L
Serum sodium and potassium analysis
Method: indirect potentiometric measurement with ion-selective electrodes. Samples: Serum. Procedure Samples were allowed to reach room temperature (calibrators, controls, serum). Samples were installed immediately before testing to minimize evaporation. Calibrators, quality control (QC), and samples were thoroughly mixed before pipetting. For a calibration run, black calibrator racks were used. Barcoded calibrators were used and placed in an unassigned black calibrator rack. To run QC, the white QC racks were used. The Gray sample rack was used to run samples and the empty sample cups were placed onto barcode labeled 13 × 75 tubes in gray sample racks and serum samples of about 150 μL were allowed to pipette into the sample cup. Samples were made to Pipette at 20-25 at a time and immediately the racks were placed on the input buffer tray.
Statistical analysis
The mean ± SEM values were calculated for each group to determine the significance of intergroup differences. Each parameter was analyzed separately using a one-way analysis of variance (ANOVA). But to find the difference between the groups, Student ‘s T’ test was used. p values < 0.05 was considered to be significant.
Ethical approval
Ethical approval was sought from the Ethical Committee of Edo State Ministry of Health, Benin City, Edo State to collect blood samples from pregnant and non-pregnant women attending the clinic in the Edo State University Teaching Hospital, Auchi, Etsako West Local Government Area of Edo State. Respondents were informed of the nature and scope of the study and their permission was sought before samples were collected from them. Approval No: EDSMH/23/102744.
Serum sodium and potassium electrolytes were assayed in blood samples. Body weight was measured using the clinical scale. Results were presented as mean ± standard error of the mean (SEM) and a p - value of less than 0.05 was considered statistically significant.
Serum sodium and potassium (Table 1)
| Table 1: Showing the mean values of serum sodium and potassium electrolytes of non-pregnant and pregnant women at different trimesters. | ||||
| Parameters | Non-pregnant | 1st trimester | 2nd trimester | 3rd trimester |
| Sodium ion concentration (mmol/L) | 135.3 ± 3.09 | 136.3 ± 1.55 | 139.0 ± 0.78 | 139.8 ± 0.91 |
| Potassium ion concentration (mmol/L) | 3.678 ± 0.26 | 3.679 ± 0.13 | 3.820 ± 0.11 | 3.767 ± 0.07 |
| * p < 0.05 indicates a significant difference when compared with non-pregnant | ||||
Graph of sodium ion concentration (Figure 1)
Figure 1: A bar chart showing sodium ion concentration variations across the trimesters of pregnancy. There were no significant differences at the first, second, and third trimester of pregnancy compared with control.
Graph of potassium ion concentration (Figure 2)
Figure 2: A bar showing potassium ion concentration variations across the trimesters of pregnancy. There were no significant differences at the first, second, and third trimester of pregnancy compared with control.
Body weight (Table 2)
| Table 2: Showing the mean values of body weight of non-pregnant and pregnant women at different trimesters. | ||||
| Parameters | Non-pregnant | 1st trimester | 2nd trimester | 3rd trimester |
| Body weight (kg) | 72.13 ± 2.11 | 74.73 ± 2.05 | 75.00 ± 1.72 | 81.56 ± 4.24* |
| * p < 0.05 indicates a significant difference when compared with non-pregnant | ||||
Graph of body weight (Figure 3)
Figure 3: A bar showing body weight variations across the trimesters of pregnancy. There was a significant increase in the third trimester of pregnancy compared with control, though there were no significant at first and second trimester compared with control.
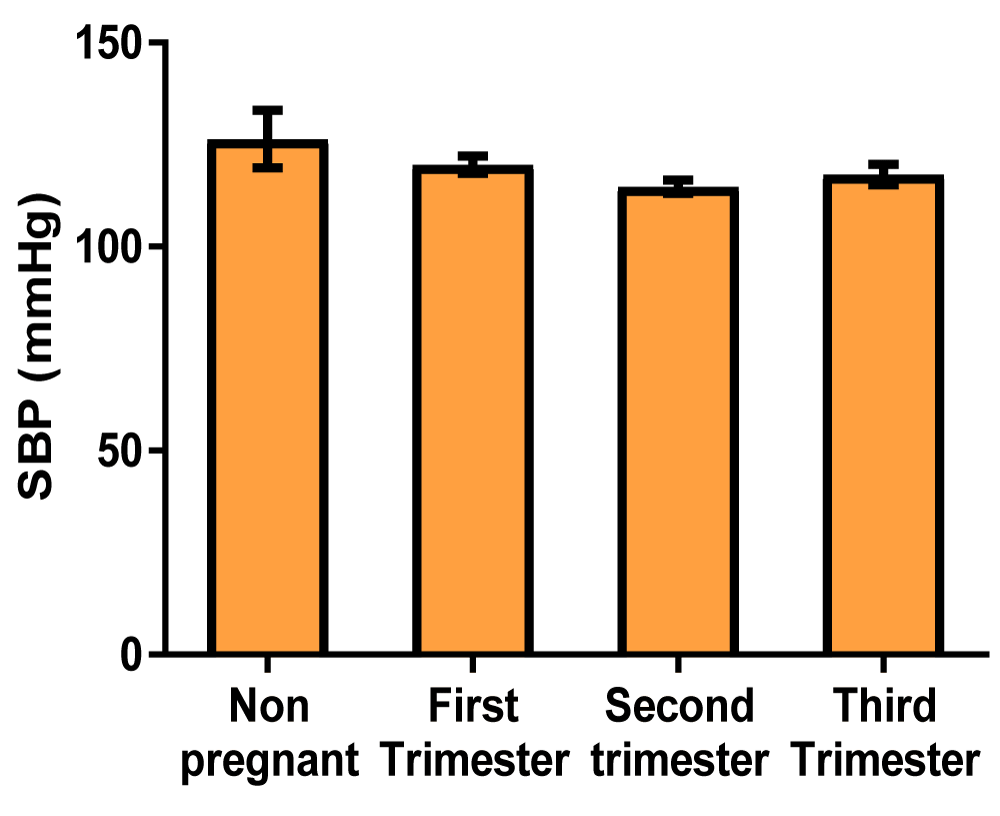
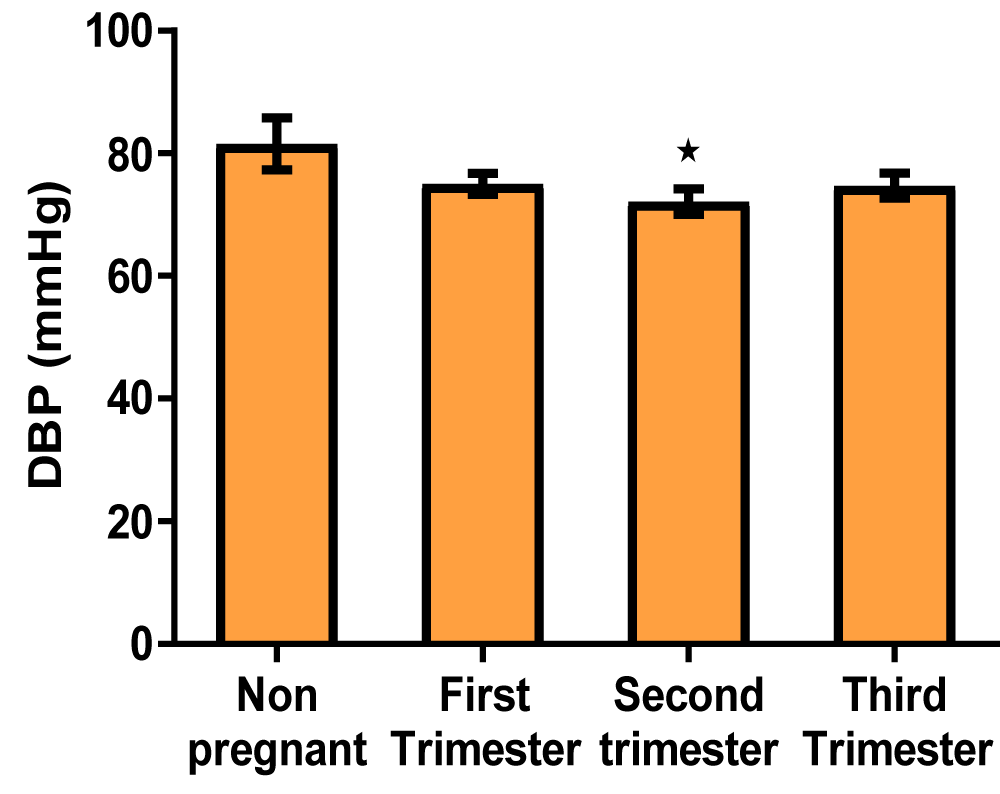
Blood pressure (Table 3)
| Table 3: Showing the mean values of blood pressure indices of non-pregnant and pregnant women at different trimesters. | ||||
| Parameters | Non-pregnant | First trimester | Second trimester | Third trimester |
| Systolic blood pressure (mmHg) | 126.3 ± 7.06 | 120.1 ± 2.17 | 114.7 ± 1.61 | 117.7 ± 2.47 |
| Diastolic blood pressure (mmHg) | 68.87 ± 2.72 | 65.40 ± 2.50 | 59.33 ± 0.89* | 59.87 ± 3.31 |
| * p < 0.05 indicates a significant difference when compared with non-pregnant | ||||
Graph of systolic blood pressure (Figure 4)
Figure 4: A bar showing systolic blood pressure variations across the trimesters of pregnancy. There were no significant differences at the first, second, and third trimester of pregnancy compared with control.
Graph of diastolic blood pressure (Figure 5)
Figure 5: A bar showing diastolic blood pressure variations across the trimesters of pregnancy. There was a significant decrease in diastolic blood pressure in the second trimester of pregnancy compared with control, but there were no significant at the first and third trimesters compared with control.
Pregnancy is a unique state characterized by adaptive alterations in the body systems, mainly caused by hormonal and metabolic effects. Some of the major changes in pregnancy include altered levels of circulating hormones, increased intravascular volume, alteration of the metabolism of food nutrients, and compression from the enlarging uterus, which are all complex physiological adaptations that are essential for the development of the fetus [43].
The result from this study showed that there was no significant difference between the serum sodium ion concentration and serum potassium ion concentration across the three trimesters of pregnancy when compared to the non-pregnant control group (Table 1 and Figures 1,2). This shows that during normal pregnancy that is devoid of complications, the serum sodium ion and serum potassium ions are adequately regulated to mask the effect of hemodilution in pregnancy that tends to alter the plasma osmolality. This regulation of serum electrolytes is well documented in the literature and it involves a feedback loop through the kidneys, adrenal glands, and hypothalamus. That when serum sodium is low, the antidiuretic hormone is suppressed and dilute urine is excreted. On the other hand, an increase in serum sodium stimulates the release of ADH, causing the kidneys to conserve water and therefore concentrated urine is excreted. Also, an atrial natriuretic peptide secreted by the heart promotes the loss of sodium through the kidney by inhibiting renin and consequently preventing aldosterone secretion. The kidney also supports this action by releasing renin, which stimulates aldosterone production, for sodium reabsorption. In addition, the renal reabsorption of sodium and potassium is tightly linked in most segments, often occurring through the same transport protein, Na-K-ATPase, that actively pumps potassium into the cell and sodium out, to maintain a serum potassium concentration. Thus, close regulation of serum sodium by the kidneys may directly ensure that serum potassium is adequately maintained within the physiological range. This, reveals that sodium and potassium homeostasis is minimally affected by estrogen and progesterone, as the kidney is capable of resisting any alteration that the pregnancy hormones may constitute. This show that the pressure of the gravid uterus on the bladder and the altered neuromuscular function of the striated sphincter which leads to urinary urgency and incontinence do not alter the serum concentrations of sodium and potassium of pregnant women [5].
There was an increase in the body weight of pregnant women across the trimesters (Table 2, Figure 3). This increase in body weight called Gestational weight gain is a unique and complex biological phenomenon that supports the functions of growth and development of the fetus. Gestational weight gain is influenced not only by changes in maternal physiology and metabolism but also by placental metabolism [44]. The weight gain during pregnancy is a factor of deposition of dietary products (protein, fat, water, and minerals), products of conception (placenta, fetus, amniotic fluid), uterus, and increase in the size/or quantity of mammary gland and blood volume. However, the products of conception contribute approximately 35 percent of the total Gestational weight gain. Another result of this work revealed that there is a gradual increase in the body weight of pregnant women from the first trimester to the second trimester. In the third trimester, this increase in body weight became significant when compared to the non-pregnant control group. One reason for this could be the decreased sensitivity of insulin, subsequently leading to the availability of almost an excess supply of glucose to the developing fetus. In their works, Catalano highlighted that over the course of pregnancy, a 40% - 60% decrease in insulin sensitivity occurs, depending on pregravid metabolic status [45]. This line of thought agrees with the report that suggested that in the last 12 weeks of pregnancy, when fetal weight increases on average from 1.0 kg to 3.5 kg, decreased insulin sensitivity increases the availability of energy to support fetal growth [46]. These alterations in maternal metabolism have generally been ascribed to placental hormones, such as human placental lactogen, progesterone, and estrogen. Also, lipid metabolism is affected as a consequence of insulin resistance during pregnancy leading to doubled or sometimes tripled concentrations of triglycerides and cholesterol late in gestation, as noticed in this work. This is in conformity with a report that an increase in fatty acids seen in pregnancy is due to an attenuated effect of insulin on lipolysis. They also added that in maternal lipid metabolism, during the anabolic phase of the first and second trimesters, the increased estrogens, progesterone, and insulin concentrations promote lipid deposition [47]. During this period, fatty acid synthesis and lipoprotein lipase expression increase, which facilitated cellular uptake of circulating triacylglycerols. Leptin may also contribute to gestational weight gain as a result of an increase in the insensitivity of the cytokine during pregnancy. This may directly lead to an increase in dietary intake during pregnancy, subsequently resulting in increased fat storage, reflecting a deviation from the physiological role of leptin in the non-pregnant state. This statement is supported by Marilyn, et al. who stated that leptin supports weight gain in pregnancy, in a feed-forward mechanism during the second half of pregnancy. Another reason for gestational weight gain could be the increased retention of body fluid by the kidney. According to the literature, this increase is mediated by the direct action of progesterone and estrogen on the kidney with the release of renin and activation of the renin-angiotensin-aldosterone mechanism, which leads to renal sodium and water retention in the distal tubule and collecting duct [48]. In addition, human chorionic gonadotropin and relaxin stimulate the release of arginine vasopressin early in pregnancy to mediate an increase in water reabsorption via aquaporin 2 channels in the collecting duct. These actions may be a direct response to an underfilled vascular system resulting from systemic vasodilatation and an increase in vascular capacitance seen in pregnancy. Thus, maternal blood volume increases by 1.5 L during pregnancy, and at term, the value increases by approximately 50% above the non-pregnant level [49].
Another important result of this work showed that there was a significant decrease in diastolic blood pressure in the second trimester of pregnancy compared with control, but there were no significant changes at the first and third trimester compared with control (Table 3, Figures 4,5). An explanation for this could be that the presence of the pregnancy hormones; progesterone and estrogen in addition to relaxin and prostaglandins triggered vasodilation by releasing nitric oxide, enhancing the flow of blood and reducing systemic vascular resistance, which in turn reduced the systolic and diastolic blood pressure, a condition called physiological hypotension [50]. This finding agrees with an earlier work, where they noticed that early in the first trimester of pregnancy, there are surges of estrogen, progesterone, and relaxin which mediates nitric oxide release leading to systemic vasodilation and a decrease in blood pressure [50]. In this study, although both the systolic and diastolic blood pressure decreased in the first and second trimesters, the values slightly increased in the third trimester of pregnancy, possibly aiming to return to the non-pregnant value.
The result of this study showed that serum sodium and potassium levels do not significantly change across the three trimesters of pregnancy in healthy women when compared to non-pregnant women, suggesting that the kidneys are well adapted to handle the serum electrolyte load during the period of pregnancy. Secondly, there was a significant increase in body weight of pregnant women in the third trimester when compared to the non-pregnant control group. This increase can be attributed to an increase in the size of the growing fetus, fluid retention, and blood volume insensitivity of insulin and leptin, which are influenced by the presence of progesterone and estrogen. Lastly, it was shown in this study that diastolic blood pressure decreases significantly in the second trimester of pregnancy compared to the control group, but there were no significant changes in the first and third trimesters compared to the control. This reduction of diastolic blood pressure is connected to the vasodilatory effect of progesterone and nitric oxide.
Novelty
The authors reaffirm that the kidneys are well adapted to handle the serum electrolyte load during the period of pregnancy. Where the kidneys are fit, the physician’s concerns and worries during pregnancy should be directed from electrolyte imbalance.
- Obrowski S, Obrowski M, Starski K. Normal Pregnancy: A Clinical. Acad. J. Ped. Neonatal. 2016; 1(1):15–18.
- Tijjani M, Abubakar E, Ibrahim AH, AIiyu SL. Effects of Pregnancy on Serum Sodium Ion and Serum Potassium Ion Concentration, Case Study of Federal Medical Centre, Nguru, Yobe State. J. of Res. in Biosci. 2014; 10:2.
- Opoku-Okrah C, Acquah BK, Dogbe EE. Changes in potassium and sodium concentrations in stored blood. Pan Afr Med J. 2015 Mar 12;20:236. doi: 10.11604/pamj.2015.20.236.5851. PMID: 27386032; PMCID: PMC4919675.
- Obembe AO, Antai AB, Ibu JO. Renal function in pregnant and non-pregnant women in Calabar – Nigeria. Niger. J. Health Biomed. Sci. 2003; 2(2):73–77.
- Omorogiuwa A, Ozor A. Electrolytes concentration patterns in the three trimesters of pregnancy. Int. J. Biol. Chem. Sci. 2015; 9(5): 2643-2647.
- Obembe AO, Antai AB. Effect of multiparity on electrolyte composition and blood pressure. Niger J Physiol Sci. 2008 Jun-Dec;23(1-2):19-22. doi: 10.4314/njps.v23i1-2.54912. PMID: 19434208.
- Shehan CL. The Wiley Blackwell Encyclopedia of Family Studies. John Wiley & Sons. 2016; 4: 406.
- Fleischman AR, Oinuma M, Clark SL. Rethinking the definition of "term pregnancy". Obstet Gynecol. 2010 Jul;116(1):136-139. doi: 10.1097/AOG.0b013e3181e24f28. PMID: 20567179.
- Charles M, Gair J. Pregnancy: Condition Information. Eunice Kennedy Shriver National Institute of Child Health and Human Development. Human Pregnancy and Birth. 2013.
- Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis. 2013 May;20(3):209-14. doi: 10.1053/j.ackd.2013.01.012. PMID: 23928384; PMCID: PMC4089195.
- Vinturache A, Khalil A. The Continuous Textbook of Women’s Medicine Series – Obstetrics Module, Fetal Development and Maternal Adaptation. Glob. Libr. Women's Med. 2021; 4:1756-2228.
- Erman A, Neri A, Sharoni R, Rabinov M, Kaplan B, Rosenfeld JB, Boner G. Enhanced urinary albumin excretion after 35 weeks of gestation and during labour in normal pregnancy. Scand J Clin Lab Invest. 1992 Sep;52(5):409-13. doi: 10.3109/00365519209088376. PMID: 1514019.
- Terry J. The major electrolytes: sodium, potassium, and chloride. J Intraven Nurs. 1994 Sep-Oct;17(5):240-7. PMID: 7965369.
- Alfarouk KO, Ahmed SBM, Ahmed A, Elliott RL, Ibrahim ME, Ali HS, Wales CC, Nourwali I, Aljarbou AN, Bashir AHH, Alhoufie STS, Alqahtani SS, Cardone RA, Fais S, Harguindey S, Reshkin SJ. The Interplay of Dysregulated pH and Electrolyte Imbalance in Cancer. Cancers (Basel). 2020 Apr 7;12(4):898. doi: 10.3390/cancers12040898. PMID: 32272658; PMCID: PMC7226178.
- Mohammed MI, Inuwa Y. Serum Sodium and Potassium Levels In Pregnant Women From Minjibir Local Government, Kano – Nigeria. J. of Pure and Applied Sci. 2010; 3(2):165–169.
- West H. Electrolytes: Definition, Functions, Imbalance, and Sources. Nutrition. 2018.
- Kamil F, Mirosława P, Dorota T, Roman T, Anna G, Sebastian P. Serum Potassium, Sodium and Calcium Levels in Healthy Individuals — Literature Review and Data Analysis. 2014; 53(1): 53–70.
- Sun Y, Mills D, Ing TS, Shapiro JI, Tzamaloukas AH. Body sodium, potassium and water in peritoneal dialysis-associated hyponatremia. Perit Dial Int. 2014 May;34(3):253-9. doi: 10.3747/pdi.2012.00201. PMID: 24863873; PMCID: PMC4033325.
- Metheny NM. Fluid and electrolyte balance: Nursing considerations. Jones & Sudbury, MA: Bartlett Learning. 5th Ed. 2012; 3.
- McCann JAS. Understanding Diseases: Nursing The Series For Clinical Excellence. Hager and Mills. 2008; 592-5.
- Chun TY, Bankir L, Eckert GJ, Bichet DG, Saha C, Zaidi SA, Wagner MA, Pratt JH. Ethnic differences in renal responses to furosemide. Hypertension. 2008 Aug;52(2):241-8. doi: 10.1161/HYPERTENSIONAHA.108.109801. Epub 2008 Jul 7. PMID: 18606909.
- Bonfils PK, Damgaard M, Taskiran M, Goetze JP, Norsk P, Gadsbøll N. Impact of diuretic treatment and sodium intake on plasma volume in patients with compensated systolic heart failure. Eur J Heart Fail. 2010 Sep;12(9):995-1001. doi: 10.1093/eurjhf/hfq100. Epub 2010 Jul 8. PMID: 20615919.
- Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009 Dec;114(6):1326-1331. doi: 10.1097/AOG.0b013e3181c2bde8. Erratum in: Obstet Gynecol. 2010 Feb;115(2 Pt 1):387. PMID: 19935037.
- Kugler JP, Hustead T. Hyponatremia and hypernatremia in the elderly. Am Fam Physician. 2000 Jun 15;61(12):3623-30. PMID: 10892634.
- Felver L. Fluid and electrolyte homeostasis and imbalances. Pathophysiology. 6th Ed. St. Louis: Elsevier. 2018; 521–40.
- Vander A, Sherman J, Luciano D. Human physiology — The Mechanisms of Body Function. 8th ed. McGraw-Hill, Boston. 2001.
- Morth JP, Pedersen BP, Toustrup-Jensen MS, Sørensen TL, Petersen J, Andersen JP, Vilsen B, Nissen P. Crystal structure of the sodium-potassium pump. Nature. 2007 Dec 13;450(7172):1043-9. doi: 10.1038/nature06419. PMID: 18075585.
- Sadava D, Heller HC, Orians GH, Purves WK, Hillis DM. Life: The Science of Biology. Gordonsville: Sinauer Associates. 2008; 10-124.
- Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011 Nov;22(11):1981-9. doi: 10.1681/ASN.2011040414. Epub 2011 Oct 6. PMID: 21980112; PMCID: PMC3231780.
- Udensi UK, Tchounwou PB. Potassium Homeostasis, Oxidative Stress, and Human Disease. Int J Clin Exp Physiol. 2017;4(3):111-122. doi: 10.4103/ijcep.ijcep_43_17. PMID: 29218312; PMCID: PMC5716641.
- Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015 Jan 6;21(1):39-50. doi: 10.1016/j.cmet.2014.12.006. PMID: 25565204; PMCID: PMC4332769.
- Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005 Dec 30;280(52):42685-93. doi: 10.1074/jbc.M510042200. Epub 2005 Oct 31. PMID: 16263722.
- Youn JH. Gut sensing of potassium intake and its role in potassium homeostasis. Semin Nephrol. 2013 May;33(3):248-56. doi: 10.1016/j.semnephrol.2013.04.005. PMID: 23953802; PMCID: PMC3748407.
- Foley K, Boguslavsky S, Klip A. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry. 2011 Apr 19;50(15):3048-61. doi: 10.1021/bi2000356. Epub 2011 Mar 25. PMID: 21405107.
- Reyes N, Gadsby DC. Ion permeation through the Na+,K+-ATPase. Nature. 2006 Sep 28;443(7110):470-4. doi: 10.1038/nature05129. PMID: 17006516.
- Baltazar RF. Hypokalaemia. In: McMillan L, editor. Basic and Bedside Electrocardiography: Lippincott Williams & Wilkins. 2009; 407.
- Pallavi PC, Pranay AJ, Jasmin HJ. Changes in serum calcium and Magnesium level in preeclampsia vs normal pregnancy. International J. of Biomedical and Advance Research. 2012; 3(6): 511-513.
- Indumati V, Kodliwadmath MV, Sheela MK. The Role of Serum Electrolytes in Pregnancy Induced Hypertension. J. of Clin. and Diagnostic Res. 2011; 5(1):66-69.
- Faisal AR, Ali R, Maha MB, Tariq HK. Sodium imbalance in preeclampsia. Iraqi. J. Med. Sci. 2009; 1:41-48.
- Sullivan CA, Martin JN Jr. Sodium and pregnancy. Clin Obstet Gynecol. 1994 Sep;37(3):558-73. doi: 10.1097/00003081-199409000-00009. PMID: 7955644.
- Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol. 2013;38(1):50-7. doi: 10.1159/000351804. Epub 2013 Jun 26. PMID: 23817179.
- Diorgu F, Friday CN. The concentration of sodium, potassium, chloride, and calcium across the trimester of pregnancy. M. O. J Women’s Health. 2021; 10(1):1‒3.
- Motosko CC, Bieber AK, Pomeranz MK, Stein JA, Martires KJ. Physiologic changes of pregnancy: A review of the literature. Int J Womens Dermatol. 2017 Oct 21;3(4):219-224. doi: 10.1016/j.ijwd.2017.09.003. PMID: 29234716; PMCID: PMC5715231.
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain during Pregnancy: Reexamining the Guidelines. Rasmussen KM, Yaktine AL, editors. Washington (DC): National Academies Press (US); 2009. PMID: 20669500.
- Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017 Feb 8;356:j1. doi: 10.1136/bmj.j1. PMID: 28179267; PMCID: PMC6888512.
- Frank H, Geoffrey C. Clinical Physiology in Obstetrics Subsequent Edition Reproductive Medicine & Technology. 1991; 486:296.
- Parrettini S, Caroli A, Torlone E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front Endocrinol (Lausanne). 2020 Nov 30;11:611929. doi: 10.3389/fendo.2020.611929. PMID: 33424775; PMCID: PMC7793966.
- Lumbers ER, Pringle KG. Roles of the circulating renin-angiotensin-aldosterone system in human pregnancy. Am J Physiol Regul Integr Comp Physiol. 2014 Jan 15;306(2):R91-101. doi: 10.1152/ajpregu.00034.2013. Epub 2013 Oct 2. PMID: 24089380.
- Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. JAMA. 2000 Nov 22-29;284(20):2611-7. doi: 10.1001/jama.284.20.2611. PMID: 11086368.
- Stephanie B, Andrei B. Hypertension in pregnancy: Pathophysiology and treatment. https://orcid.org/0000-0003-1339 3934 [email protected] all authors and affiliations https://doi.org/10.1177/205031211984 April 10, 2019