More Information
Submitted: June 13, 2023 | Approved: June 20, 2023 | Published: June 21, 2023
How to cite this article: Guven S, Comert EH, Guven ESG, Demir B, Karcaaltincaba D. Amniotic Fluid Ischemia Modified Albumin as a Novel Prenatal Diagnostic Marker for Down Syndrome: A Prospective Case-Control Study. Clin J Obstet Gynecol. 2023; 6: 082-087.
DOI: 10.29328/journal.cjog.1001133
Copyright License: © 2023 Guven S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Amniocentesis; Biochemical test; Down Syndrome; Prenatal screening
Abbreviations: IMA: Ischemia-Modified Albumin; HGF: Hepatocyte Growth Factor; ABSU: Absorbance Unit; ROC: Receiver Operating Curve; CVS: Chorionic Villus Sampling; ELISA: Enzyme Link Immunoassay; AF: Amniotic Fluid; SOD: Superoxide Dismutase; IUGR: Intrauterine Growth Restriction
Amniotic Fluid Ischemia Modified Albumin as a Novel Prenatal Diagnostic Marker for Down Syndrome: A Prospective Case-Control Study
Suleyman Guven1*, Erhan Huseyin Comert1, Emine Seda Guvendag Guven1, Bulent Demir2 and Deniz Karcaaltincaba3
1Department of Obstetrics and Gynecology, Karadeniz Technical University Faculty of Medicine, Trabzon, Turkey
2Department of Obstetrics and Gynecology, Onsekiz Mart University Faculty of Medicine, Canakkale, Turkey
3Department of Obstetrics and Gynecology, Gazi University Faculty of Medicine, Trabzon, Turkey
*Address for Correspondence: Suleyman Guven, Department of Obstetrics and Gynecology, Karadeniz Technical University Faculty of Medicine, Postcode 61080, Trabzon, Turkey, Email: [email protected]
Aims: There is no study in the literature about ischemia-modified albumin (IMA) and hepatocyte growth factor (HGF) levels in amniotic fluid for Down syndrome cases. The aim of this study was to investigate the changes of IMA and HGF in Down syndrome cases at 16-20 weeks of gestation compared to normal fetuses.
Methods: For this prospective case-control study, following reaching the number of 20 women (study group) who had the prenatal diagnosis of Down syndrome, maternal and gestational age-matched pregnant women with normal constitutional karyotype were selected for the control group (n = 74) from the stored amniotic fluid samples.
Results: Mean women and gestational ages were comparable between the two groups. Amniotic fluid IMA (1.32 ± 0.13 vs. 1.11 ± 0.11 ABSU, respectively, p < 0.001) and HGF (2743.53 ± 1389.28 vs. 2160.12 ± 654.63 pg/mL, respectively, p = 0.008). Levels were significantly higher in pregnant women having Down syndrome fetuses compared with women having normal fetuses. The amniotic fluid IMA levels for the diagnosis of Down syndrome, and the sensitivity and specificity were calculated as 95.0% and 71.6% for the limit value 1.171 cm3, respectively.
Conclusion: In cases with suspected Down syndrome, the diagnosis of Down Syndrome may be made in approximately 1 hour with high sensitivity and specificity by measuring the IMA level in the amniotic fluid sample taken for fetal karyotyping.
The most common fetal aneuploidy in pregnancy is trisomy 21 (Down Syndrome), which is a frightening dream for both the mother and the clinician. Fetal aneuploidy screening during pregnancy is one of the routinely recommended tests. Many current screening and diagnostic tests have been defined for trisomy 21, which is seen in an average of 700 live births. Although screening tests do not make a definitive diagnosis, when they give a high-risk result, this result needs to be confirmed with a diagnostic test. While another limitation of screening tests is that they can give false positive test results, the most important disadvantage of diagnostic tests is that they create a small risk of procedural miscarriage [1].
Down syndrome is the most common chromosomal abnormality, which is the incidence estimated between one in 1000 to one in 700 live births and no significant differences between societies. But, if we take into account medically induced abortions and stillborns, it is increased to approximately one in 450 births [2]. Assisted reproductive techniques and advanced-age pregnancies have increased in recent years. This situation caused the pregnancy to shift to advanced ages and naturally caused an increase in Down syndrome cases. For this reason during the last 20 years, an increase of 10% in the number of pregnancies with Down syndrome has been noticed [3].
For trisomy 21, one of the most common genetic syndromes in pregnancy, many screening tests have been described for years. The basic screening tests used today are maternal serum screening tests (double test, triple test, quadruple test) and diagnostic tests (chorionic villus sampling (CVS), amniocentesis, cordocentesis) [2]. Apart from these, the non-invasive prenatal screening test (NIPT) with the highest sensitivity is another screening test. All screening tests have a 5% - 10% false positive risk, and diagnostic tests have an average of 1% fetal loss risk. All these rates pave the way for the search for a fast, effective, and highly sensitive screening test in prenatal Down Syndrome screening [4].
Although the main role of cytokine change in pregnancy has not been shown, it is known to be important in fetal growth and development [5]. There are studies reporting that some fetal complications are also associated with the intrauterine oxidative stress process [6,7]. In this context, the oxidative stress process varies in fetal Down syndrome compared to healthy pregnancies [8].
In a previously published study, we reported that the serum IMA value increased during pregnancy [9]. Similarly, we reported that amniotic fluid cytokine and IMA levels changed in cases with false positive first-second trimester biochemical Down Syndrome screening test results [10]. All these studies have led us to the idea that amniotic fluid cytokine and IMA levels will also be affected in real Down Syndrome cases and this can be used in the prenatal diagnosis of Down Syndrome. For all these reasons, a research plan was made in this way.
Biochemical markers with high sensitivity and rapid results are required in the diagnosis of Down syndrome. Because fetal karyotype analysis, which is a definitive diagnostic test, gives results within at least 21 days. There is no study in the literature about IMA and HGF levels in amniotic fluid for Down syndrome cases. This prospective study was planned to investigate the change of IMA and HGF in Down syndrome cases compared to normal fetuses.
Totally 94 amniotic fluid samples from women who had confirmed intrauterine live fetuses at 16-20 weeks of gestation were included in this prospective case-control study. The study was conducted in a high-risk pregnancy unit of a tertiary hospital for a one-year duration. Totally 94 women were included. Following reaching the number of 20 women (study group) who had the diagnosis of Down syndrome, maternal and gestational age-matched pregnant women with normal constitutional karyotypes were selected for the control group (n = 74) from the stored amniotic fluid samples. Only singleton pregnancies were included. Women having a history of acute or chronic systemic disease (endocrinologic, cardiovascular, pulmonary, or urinary system diseases) and taking any medication other than vitamin and iron supplements, and having any type of anomalous fetus were not included.
During transabdominal amniocentesis for various indications (abnormal serum first or second trimester Down syndrome screening test result or advanced maternal age etc.), all amniotic fluid samples were stored during two years of the study. Almost 2 mL - 3 mL clean amniotic fluid sample which was not used for the amniotic fluid genetic analysis was centrifuged at 900 g for 10 min to remove the supernatant and then stored at –80 0C until final analysis.
Reduced cobalt to albumin-binding capacity (IMA level) was analyzed using the rapid and colorimetric method developed by Bar-Or and co-workers [11]. In our laboratory, the albumin-binding capacity assay within-run percent coefficient of variation (CV%) of women samples averaged 2.1% [12]. IMA has a short half-life, returning to baseline values in 6 h – 12 h [13].
Amniotic fluid hepatocyte growth was measured with commercially available ELISA kits. HGF (catalog number ELH-HGF-001, RayBiotech, Inc, Norcross, USA) concentrations were measured by ELISA. The intra- and inter-assay coefficients of variation were as follows: < %10% and < %12%, respectively
Student t-test or Mann-Whitney U-test (in case of non-parametric distribution) was used for the comparison of amniotic fluid IMA levels and clinical characteristics between the study and control groups. The correlation coefficient was determined by Pearson’s test. Statistical analyses were performed with SPSS (version 13.0). Statistical significance was set at p - 0.05. Amniotic fluid IMA levels are expressed in ABSU (absorbance unit), and amniotic fluid HGF levels were expressed as pg/mL.
Institutional ethics board approval was obtained for this case-control study (Approval number: 2012/24).
G-power computer-assisted sample size calculation program was used for sample size calculation. Based on our preliminary study results following givens effect size d = 1, α err prob = 0.05, Power (1-β err prob) = 0.95, Allocation ratio N2/N1=3 were accepted. The calculated total minimum number was 60 (N1 (Down syndrome group) = 15, N2 (Control group) = 45).
Mean women's age (35.50 ± 6.40 vs. 33.61 ± 6.61, respectively) were comparable between the study and control groups. The comparison of clinical characteristics of the pregnant women having Down syndrome fetuses (study group) compared with women having normal fetuses (control group) was given in Table 1.
| Table 1: Comparison of the clinical characteristics of the pregnant women having down syndrome fetus (study group) compared with women having normal fetus (control group). | |||
| Clinical characteristics | Study group (n = 20) |
Control group (n = 74) |
p |
| Age (year) | 35.50 ± 6.40 | 33.61 ± 6.61 | 0.256a |
| Gravida (no.) | 3.38 ± 1.98 | 2.20 ± 1.30 | 0.007b |
| Parity (no.) | 1.84 ± 1.52 | 0.95 ± 1.15 | 0.008b |
| Gestational age (week) | 17.91 ± 1.45 | 17.50 ± 1.33 | 0.262b |
| Values are given as mean and standard deviation aStudent- t - test, bMan-Whitney U test were used for comparison. | |||
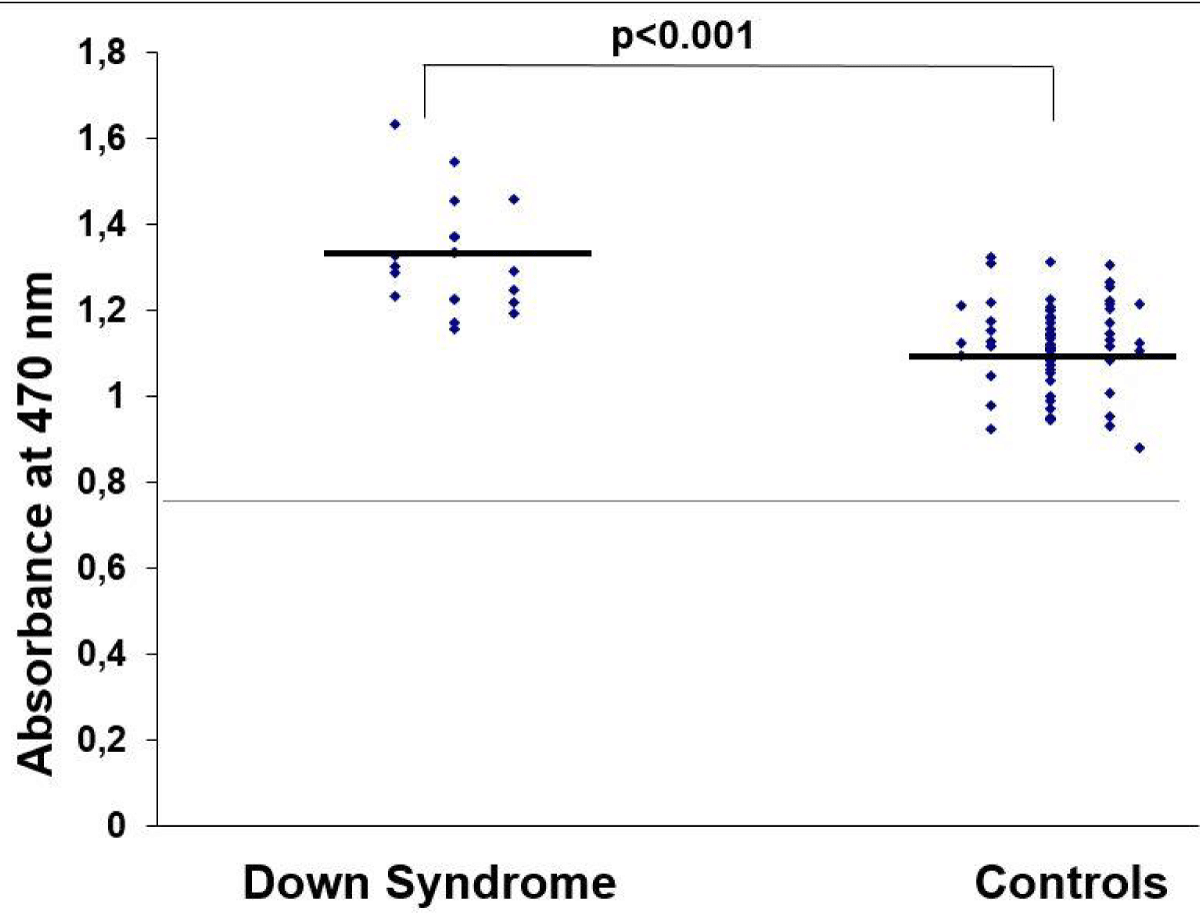
Amniotic fluid IMA levels were significantly higher in pregnant women having Down syndrome fetuses compared with women having normal fetuses (1.32 ± 0.13 vs. 1.11 ± 0.11 ABSU, respectively, p < 0.001, Student t - test, Figure 1). Furthermore, amniotic fluid HGF levels were significantly higher in pregnant women having Down syndrome fetuses compared with women having normal fetuses (2743.53 ± 1389.28 vs. 2160.12 ± 654.63 pg/mL, respectively, p = 0.008, Student t - test).
Figure 1: Comparison of amniotic fluid IMA in pregnant women having Down syndrome fetuses compared with women having normal fetuses.
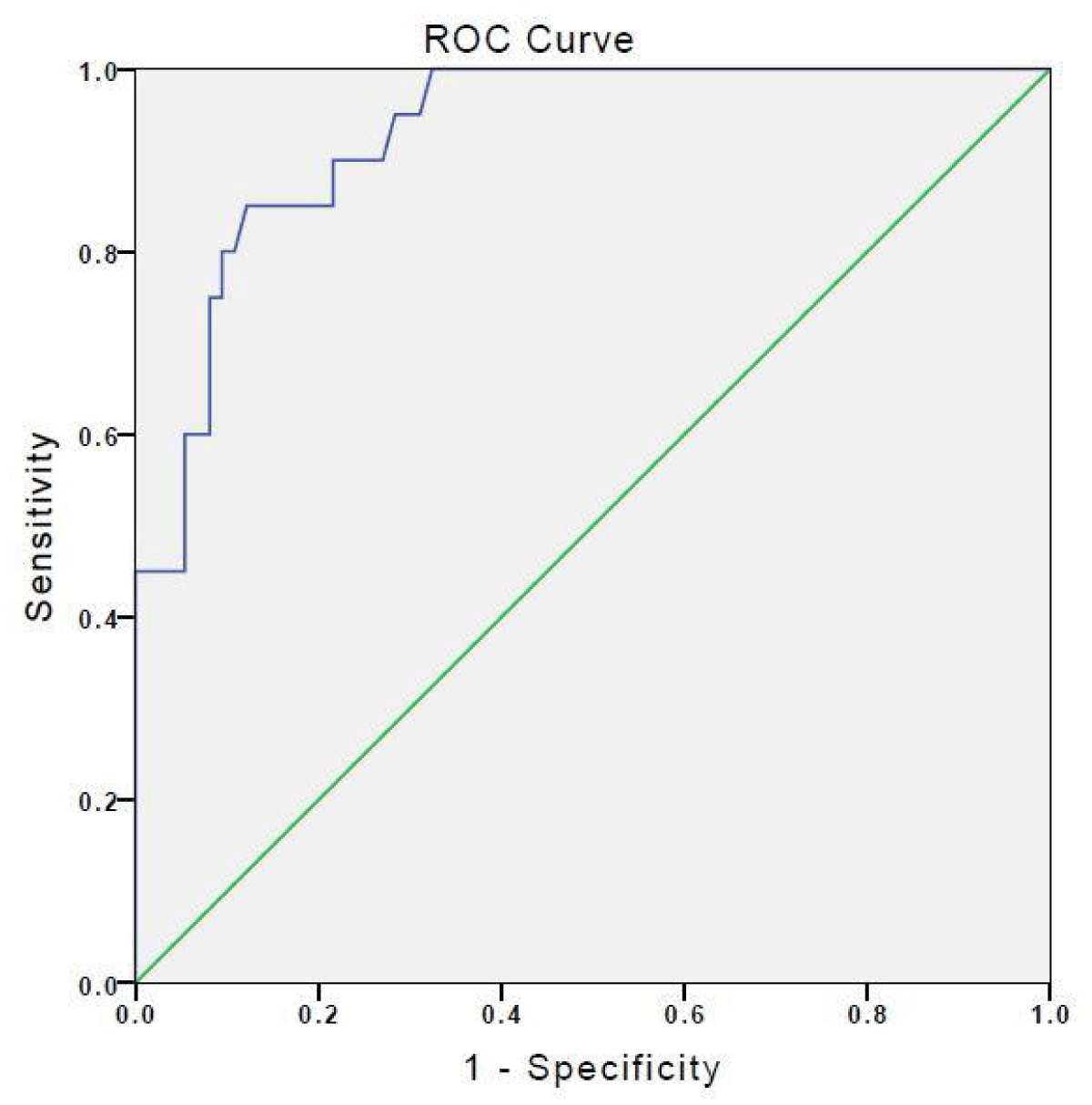
Considering the prenatal diagnosis of Down Syndrome in the whole group, ROC analysis showing the relationship between amniotic fluid IMA levels in the second trimester and Down syndrome was given in Figure 2. The amniotic fluid IMA levels for the diagnosis of Down syndrome, and the sensitivity and specificity were calculated as 95.0% and 71.6% for the limit value 1.171 cm3 (AUC 92.9%, p < 0.001, 95% CI 0.876-0.982), respectively. A positive correlation was found between IMA and HGF levels (r = 0.241, p = 0.019).
Figure 2: ROC analysis showing the relationship between amniotic fluid IMA and fetal Down syndrome.
Biochemical test analysis from the amniotic fluid is a relatively new approach in the prenatal diagnosis of Down syndrome. According to the results of the research, the level of IMA in the amniotic fluid of Down syndrome cases was found to be statistically significantly higher than in cases with normal euploid karyotype.
The NIPT test yields result within an average of 10 days, while the diagnostic test CVS or amniocentesis gives definite results in at least 21 days. During this period, the patient's anxious expectation increases. It is clear that there is a need for biochemical tests that can reduce the anxiety of the patient, give results within a few hours, and can be performed immediately in the same clinic. Our study, which measured the IMA and HGF levels in amniotic fluid and yielded significant results, may be useful in eliminating this deficiency in the literature.
The role of cytokines in human pregnancy is not exactly understood [5]. The cytokine interactions between the fetoplacental unit and the maternal immune system are important for fetal development and growth [6,7]. Several cytokines are detectable in human Amniotic fluid (AF), such as HGF, interleukins, GM-CSF, TNFα (tumor necrosis factor-alpha) and IFNγ (interferon gamma). The level of cytokines may alter with gestation, the onset of labor, infection, etc. in AF [10,14-20]. A small number of studies had also linked amniotic fluid HGF, IMA, and interleukins to fetal growth, ischemia, and/or inflammation processes [10,20].
There are research results showing that the fetal oxidative stress process is affected in fetuses with Down syndrome. One of the most important pieces of evidence of the influence process is the change in the gene expression of the SOD-1 (superoxide dismutase-1) enzyme, which has an important role in the oxidative stress process located on the 21st chromosome [21]. It was reported that this enzyme level was higher in amniotic fluid samples obtained from cases with Down syndrome compared to healthy pregnancies [22,23]. It has also been reported that increased SOD-1 enzyme activity augments the oxidative stress process, resulting in increased hydrogen peroxide levels [24-26].
The binding capacity of the modified N-terminal end of albumin decreases the transition of metals such as Cu+2, Co+2, and Ni+2 in free radical damage, free iron and copper exposure, energy-related membrane damage, hypoxia, and acidosis [27-29]. This modified form of albumin is called ischemia-modified albumin (IMA) [30]. IMA occurs not only in ischemic heart diseases but also in different ischemia models affecting other organs due to high oxidative stress [27]. Serum IMA levels increase in arthroscopic knee surgery, systemic sclerosis, skeletal muscle ischemia after exercise, diabetes mellitus, liver diseases, some cancers, infection, and peripheral vascular diseases in non-cardiac ischemic diseases [30]. Oxidative stress biomarkers in scleroderma patients also increased and IMA levels were found to be high [31]. The increase in SOD-1 level, which was a marker of oxidative stress in Down syndrome cases, suggested that Down syndrome cases were exposed to higher oxidative stress in the intrauterine period. This situation supports the hypothesis that the measurement of amniotic fluid IMA level, which is an easily measurable indicator of oxidative stress, may be an effective biomarker in the diagnosis of Down syndrome. IMA, which was found to be high in amniotic fluid in our study, also supported this argument.
HGF is abundant in the placenta and is important in the process of placental fetal growth. Although it has not been defined as an important indicator in IUGR cases in the literature [32], it has been reported to be associated with the development of macrosomia, affecting lipid metabolism [33], liver regeneration, and immunomodulation processes [34]. According to another current experimental study results, HGF; In case of acute liver failure, it reduces endoplasmic reticulum stress by showing an antiapoptotic effect. In addition, in the case of acetaminophen-mediated liver damage, an antiapoptotic and antioxidant effect was created by amniotic fluid-derived HGF therapy. In this way, it can be concluded that HGF may have the potential to correct oxidative stress-mediated damage [35,36].
According to our extensive literature research, no research could be found regarding the change of HGF in the amniotic fluid in Down's syndrome cases. Only one case partially related to the subject was reported. High serum HGF level in Down syndrome cases with the transient myeloproliferative disease also supports the argument that HGF may be a separate biomarker in Down syndrome cases.
Increased HGF levels in Down syndrome cases can be evaluated within the scope of a protective response to the existing increased intrauterine oxidative stress (due to increased amniotic fluid IMA level) [37].
Down syndrome (trisomy 21), has an immunological deficiency. These patients frequently present with three clinical findings; increased susceptibility to infection, increased risk of malignancy, and increased incidence of autoimmune diseases due to developing autoantibodies. Based on one study result, there are abnormalities in the humoral and cellular response levels in these patients [38]. T cell maturation of the thymus gland was impaired in patients with Down syndrome than in normal fetuses. Cellular immunity is markedly affected and CD4 / CD8 ratio is decreased [39]. There are literature data showing that HGF is involved in the immunomodulation process [34]. In our study, the increased amniotic fluid HGF level in Down syndrome cases may be protective purposes within the scope of correcting the existing immunologic defect in Down syndrome.
As a result of our study, we reported that IMA was a marker that could be used in the prenatal diagnosis of Down syndrome, not HGF. The weak correlation coefficient was related to the change in IMA and HGF. We thought that the IMA and HGF changes were related to each other, but due to the weaknesses of our study, we could not demonstrate this with a good correlation coefficient. For this, further studies with large series are needed.
The most important limiting aspect of our study was that it included a low number of Down syndrome cases. In addition, the lack of molecular data on cytokines was another limiting aspect of the study. Further studies including larger series and molecular study results may confirm the accuracy of our data and support our hypothetical mechanism.
In our study, it was stated that a possible oxidative stress-mediated mechanism may prevail in cases of Down's syndrome. In this context, the high level of IMA in amniotic fluid may be useful in the prenatal diagnosis of Down syndrome. In cases with suspected Down syndrome, the diagnosis of Down Syndrome may be made in approximately 1 hour with high sensitivity and specificity by measuring the IMA level in the amniotic fluid sample taken for fetal karyotyping.
The most important clinical use of the study was that it predicted the diagnosis of prenatal Down Syndrome with high sensitivity in a short time. In other words, the couple with high anxiety can be informed about whether their baby is Down by measuring the level of amniotic fluid IMA with 90% sensitivity without waiting for 21 days for a definitive result.
- Cuckle H, Benn P. Review of epidemiological factors (other than maternal age) that determine the prevalence of common autosomal trisomies. Prenat Diagn. 2021 Apr;41(5):536-544. doi: 10.1002/pd.5822. Epub 2020 Sep 21. PMID: 32895968.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics; Committee on Genetics; Society for Maternal-Fetal Medicine. Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin, Number 226. Obstet Gynecol. 2020 Oct;136(4):e48-e69. doi: 10.1097/AOG.0000000000004084. PMID: 32804883.
- Loane M, Morris JK, Addor MC, Arriola L, Budd J, Doray B, Garne E, Gatt M, Haeusler M, Khoshnood B, Klungsøyr Melve K, Latos-Bielenska A, McDonnell B, Mullaney C, O'Mahony M, Queisser-Wahrendorf A, Rankin J, Rissmann A, Rounding C, Salvador J, Tucker D, Wellesley D, Yevtushok L, Dolk H. Twenty-year trends in the prevalence of Down syndrome and other trisomies in Europe: impact of maternal age and prenatal screening. Eur J Hum Genet. 2013 Jan;21(1):27-33. doi: 10.1038/ejhg.2012.94. Epub 2012 Jun 20. PMID: 22713804; PMCID: PMC3522199.
- Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H, Tomlinson MW, Pereira L, Spitz JL, Hollemon D, Cuckle H, Musci TJ, Wapner RJ. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015 Apr 23;372(17):1589-97. doi: 10.1056/NEJMoa1407349. Epub 2015 Apr 1. PMID: 25830321.
- Tabibzadeh S. Cytokines and the hypothalamic-pituitary-ovarian-endometrial axis. Hum Reprod. 1994 May;9(5):947-67. doi: 10.1093/oxfordjournals.humrep.a138621. PMID: 7929746.
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993 Jul;14(7):353-6. doi: 10.1016/0167-5699(93)90235-D. PMID: 8363725.
- Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997 Oct;18(10):478-82. doi: 10.1016/s0167-5699(97)01127-4. PMID: 9357139.
- Nanji AA, Khwaja S, Tahan SR, Sadrzadeh SM. Plasma levels of a novel noncyclooxygenase-derived prostanoid (8-isoprostane) correlate with severity of liver injury in experimental alcoholic liver disease. J Pharmacol Exp Ther. 1994 Jun;269(3):1280-5. PMID: 8014871.
- Guven S, Alver A, Mentese A, Ilhan FC, Calapoglu M, Unsal MA. The novel ischemia marker 'ischemia-modified albumin' is increased in normal pregnancies. Acta Obstet Gynecol Scand. 2009;88(4):479-82. doi: 10.1080/00016340902777517. PMID: 19235558.
- Guven S, Karahan SC, Kandemir O, Ucar U, Cora AO, Bozkaya H. Occult inflammation and/or ischemia may be responsible for the false positivity of biochemical Down syndrome screening test. J Perinat Med. 2010 Jul;38(4):367-71. doi: 10.1515/jpm.2010.062. PMID: 20297899.
- Bar-Or D, Curtis G, Rao N, Bampos N, Lau E. Characterization of the Co(2+) and Ni(2+) binding amino-acid residues of the N-terminus of human albumin. An insight into the mechanism of a new assay for myocardial ischemia. Eur J Biochem. 2001 Jan;268(1):42-7. doi: 10.1046/j.1432-1327.2001.01846.x. PMID: 11121100.
- Karahan SC, Koramaz I, Altun G, Uçar U, Topbaş M, Menteşe A, Kopuz M. Ischemia-modified albumin reduction after coronary bypass surgery is associated with the cardioprotective efficacy of cold-blood cardioplegia enriched with N-acetylcysteine: a preliminary study. Eur Surg Res. 2010;44(1):30-6. doi: 10.1159/000262324. Epub 2009 Dec 1. PMID: 19955769.
- Apple FS, Wu AH, Mair J, Ravkilde J, Panteghini M, Tate J, Pagani F, Christenson RH, Mockel M, Danne O, Jaffe AS; Committee on Standardization of Markers of Cardiac Damage of the IFCC. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem. 2005 May;51(5):810-24. doi: 10.1373/clinchem.2004.046292. Epub 2005 Mar 17. PMID: 15774573.
- Santhanam U, Avila C, Romero R, Viguet H, Ida N, Sakurai S, Sehgal PB. Cytokines in normal and abnormal parturition: elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine. 1991 Mar;3(2):155-63. doi: 10.1016/1043-4666(91)90037-e. PMID: 1888885.
- Tsunoda H, Tamatani T, Oomoto Y, Hirai Y, Kasahara T, Iwasaki H, Onozaki K. Changes in interleukin 1 levels in human amniotic fluid with gestational ages and delivery. Microbiol Immunol. 1990;34(4):377-85. doi: 10.1111/j.1348-0421.1990.tb01018.x. PMID: 2362563.
- Opsjłn SL, Wathen NC, Tingulstad S, Wiedswang G, Sundan A, Waage A, Austgulen R. Tumor necrosis factor, interleukin-1, and interleukin-6 in normal human pregnancy. Am J Obstet Gynecol. 1993 Aug;169(2 Pt 1):397-404. doi: 10.1016/0002-9378(93)90096-2. PMID: 8362955.
- Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine. 1993 Jan;5(1):81-8. doi: 10.1016/1043-4666(93)90027-3. PMID: 7683506.
- Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995 Mar;172(3):960-70. doi: 10.1016/0002-9378(95)90028-4. PMID: 7892891.
- Oláh KS, Vince GS, Neilson JP, Deniz G, Johnson PM. Interleukin-6, interferon-gamma, interleukin-8, and granulocyte-macrophage colony stimulating factor levels in human amniotic fluid at term. J Reprod Immunol. 1996 Nov;32(1):89-98. doi: 10.1016/s0165-0378(96)00990-4. PMID: 8953522.
- Horibe N, Okamoto T, Itakura A, Nakanishi T, Suzuki T, Kazeto S, Tomoda Y. Levels of hepatocyte growth factor in maternal serum and amniotic fluid. Am J Obstet Gynecol. 1995 Sep;173(3 Pt 1):937-42. doi: 10.1016/0002-9378(95)90370-4. PMID: 7573273.
- Lieman-Hurwitz J, Dafni N, Lavie V, Groner Y. Human cytoplasmic superoxide dismutase cDNA clone: a probe for studying the molecular biology of Down syndrome. Proc Natl Acad Sci U S A. 1982 May;79(9):2808-11. doi: 10.1073/pnas.79.9.2808. PMID: 6211674; PMCID: PMC346295.
- Netto CB, Siqueira IR, Fochesatto C, Portela LV, da Purificação Tavares M, Souza DO, Giugliani R, Gonçalves CA. S100B content and SOD activity in amniotic fluid of pregnancies with Down syndrome. Clin Biochem. 2004 Feb;37(2):134-7. doi: 10.1016/j.clinbiochem.2003.09.010. PMID: 14725944.
- Baeteman MA, Mattei MG, Baret A, Gamerre M, Mattei JF. Immunoreactive SOD-1 in amniotic fluid, amniotic cells and fibroblasts from trisomy 21 fetus. Acta Paediatr Scand. 1985 Sep;74(5):697-700. doi: 10.1111/j.1651-2227.1985.tb10016.x. PMID: 2931942.
- de Haan JB, Wolvetang EJ, Cristiano F, Iannello R, Bladier C, Kelner MJ, Kola I. Reactive oxygen species and their contribution to pathology in Down syndrome. Adv Pharmacol. 1997;38:379-402. doi: 10.1016/s1054-3589(08)60992-8. PMID: 8895817.
- de Haan JB, Cristiano F, Iannello RC, Kola I. Cu/Zn-superoxide dismutase and glutathione peroxidase during aging. Biochem Mol Biol Int. 1995 May;35(6):1281-97. PMID: 7492966.
- de Haan JB, Newman JD, Kola I. Cu/Zn superoxide dismutase mRNA and enzyme activity, and susceptibility to lipid peroxidation, increases with aging in murine brains. Brain Res Mol Brain Res. 1992 Apr;13(3):179-87. doi: 10.1016/0169-328x(92)90025-7. PMID: 1593944.
- Sbarouni E, Georgiadou P, Voudris V. Ischemia modified albumin changes - review and clinical implications. Clin Chem Lab Med. 2011 Feb;49(2):177-84. doi: 10.1515/CCLM.2011.037. Epub 2010 Nov 18. PMID: 21083441.
- Worster A, Devereaux PJ, Heels-Ansdell D, Guyatt GH, Opie J, Mookadam F, Hill SA. Capability of ischemia-modified albumin to predict serious cardiac outcomes in the short term among patients with potential acute coronary syndrome. CMAJ. 2005 Jun 21;172(13):1685-90. doi: 10.1503/cmaj.045194. PMID: 15967971; PMCID: PMC1150260.
- Erdem SS, Yerlikaya FH, Çiçekler H, Gül M. Association between ischemia-modified albumin, homocysteine, vitamin B(12) and folic acid in patients with severe sepsis. Clin Chem Lab Med. 2012 Feb 14;50(8):1417-21. doi: 10.1515/cclm-2011-0794. PMID: 22868807.
- Piwowar A, Knapik-Kordecka M, Warwas M. Ischemia-modified albumin level in type 2 diabetes mellitus - Preliminary report. Dis Markers. 2008;24(6):311-7. doi: 10.1155/2008/784313. PMID: 18688079; PMCID: PMC3850615.
- Borderie D, Allanore Y, Meune C, Devaux JY, Ekindjian OG, Kahan A. High ischemia-modified albumin concentration reflects oxidative stress but not myocardial involvement in systemic sclerosis. Clin Chem. 2004 Nov;50(11):2190-3. doi: 10.1373/clinchem.2004.034371. PMID: 15502098.
- Ohnishi Y, Yamashiro C, Yanagihara T, Hata T. Hepatocyte growth factor concentration in the early second-trimester amniotic fluid does not predict fetal growth at birth. Hum Reprod. 1999 Oct;14(10):2625-8. doi: 10.1093/humrep/14.10.2625. PMID: 10527998.
- Visiedo F, Bugatto F, Carrasco-Fernández C, Sáez-Benito A, Mateos RM, Cózar-Castellano I, Bartha JL, Perdomo G. Hepatocyte growth factor is elevated in amniotic fluid from obese women and regulates placental glucose and fatty acid metabolism. Placenta. 2015 Apr;36(4):381-8. doi: 10.1016/j.placenta.2015.01.199. Epub 2015 Feb 7. PMID: 25690371.
- Maraldi T, Beretti F, Guida M, Zavatti M, De Pol A. Role of hepatocyte growth factor in the immunomodulation potential of amniotic fluid stem cells. Stem Cells Transl Med. 2015 Jun;4(6):539-47. doi: 10.5966/sctm.2014-0266. Epub 2015 Apr 14. PMID: 25873747; PMCID: PMC4449098.
- Sahan OB, Gunel-Ozcan A. Hepatocyte Growth Factor and Insulin-like Growth Factor-1 based Cellular Therapies for Oxidative Stress Injury. Curr Stem Cell Res Ther. 2021;16(7):771-791. doi: 10.2174/1574888X16999201124153753. PMID: 33238860.
- Cheng W, Liu GP, Kong D, Huang W, Sun Y, Zhao D. Downregulation of miR-1224 protects against oxidative stress-induced acute liver injury by regulating hepatocyte growth factor. J Cell Biochem. 2019 Aug;120(8):12369-12375. doi: 10.1002/jcb.28502. Epub 2019 Mar 8. PMID: 30848506.
- Hirono K, Miura M, Kanegane H, Miyamoto M, Yoshimura N, Ichida F, Ito E, Miyawaki T. Hepatocyte growth factor in transient myeloproliferative disorder of Down syndrome. Pediatr Int. 2009 Oct;51(5):754-5. doi: 10.1111/j.1442-200X.2009.02897.x. PMID: 19799747.
- Douglas SD. Down syndrome: immunologic and epidemiologic associations-enigmas remain. J Pediatr. 2005 Dec;147(6):723-5. doi: 10.1016/j.jpeds.2005.09.002. PMID: 16356417.
- de Hingh YC, van der Vossen PW, Gemen EF, Mulder AB, Hop WC, Brus F, de Vries E. Intrinsic abnormalities of lymphocyte counts in children with down syndrome. J Pediatr. 2005 Dec;147(6):744-7. doi: 10.1016/j.jpeds.2005.07.022. PMID: 16356423.