More Information
Submitted: May 31, 2023 | Approved: June 15, 2023 | Published: June 16, 2023
How to cite this article: Chanda K, Lian LS, Yan KY, Qian H, Abulikem G, et al. Racial and Ethnic Disparities in Pregnancy-related Complications: Findings at Mansa General Hospital and 2nd Affiliated Hospital of Nanjing Medical University. Clin J Obstet Gynecol. 2023; 6: 065-075.
DOI: 10.29328/journal.cjog.1001131
Copyright License: © 2023 Chanda K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Ethnic and race; Pregnancy-related complications; Maternal health
Abbreviations: GBS: Group B streptococcal; GDM: Gestation Diabetes Mellitus; HBV: Hepatitis B Virus; HDP: Hypertensive Disorders of Pregnancy; IHC: Intra Hepatic Cholestasis; MGH: Mansa General Hospital; NMU: Nanjing Medical University; Olig: Oligohydramnios; OR: Odds Ratio; PIH: Pregnancy Induced Hypertension; PPROM: Preterm Premature Rupture of Membranes; PRC: People’s Republic of China; PROM Premature Rupture of Membranes; SPSS: Statistical Package for the Social Sciences; SSA: Sub-Saharan Africa; UTH: University Teaching Hospital; UTI: Urinary Tract Infection; WHO: World Health Organization
Racial and Ethnic Disparities in Pregnancy-related Complications: Findings at Mansa General Hospital and 2nd Affiliated Hospital of Nanjing Medical University
Kasonde Chanda1, Liang Sheng Lian2, Kong Yi Yan2, Huang Qian2, Gulidiya Abulikem1, Royd Nkalamo Nonde1 and Ying Xiao Yan2*
1Department of Obstetrics and Gynecology, Mansa General Hospital, Mansa District, Luapula Province, Zambia
2Department of Obstetrics and Gynecology, 2nd Affiliated Hospital of Nanjing Medical University, Nanjing City, Jiangsu Province, PR China
*Address for Correspondence: Ying Xiao Yan, Professor, Department of Obstetrics and Gynecology, 2nd Affiliated Hospital of Nanjing Medical University, Jiangsu Province, PR China, Email: [email protected]
Background: 800 women die and 2.6 million stillbirths occur worldwide related to pregnancy complications. Racial/ethnic disparities in pregnancy-related mortality have continued to be significantly higher among black than whites due to various factors. We sought to investigate complications among pregnant women of different race/ethnicity.
Methods: Cross-sectional observational study of 2030 obstetric cases randomly selected for the period January 1 to December 31, 2021. Data was collected from the hard copy and electronic inpatients’ records. Analysis was performed using SPSS version 23. Descriptive statistics analyzed the pregnancy complication frequencies, standard deviations, range, minimum and maximum values. Maternal characteristics were analyzed using an independent samples t-test. Maternal characteristics were evaluated using the two samples t-test. The odds ratios and confidence intervals were calculated as measures of association between ethnicity/race and pregnancy complications using a binary logistic regression model. Confidence interval was set at 95% and p < 0.05 (2-tailed) was considered statistically significant.
Results: 76.25% of Chinese and 67.86% of Zambians were affected by one or more complications. The mean ± standard deviation for MGH [age (26.69 ± 7.33), gravidity (3.35 ± 2.08), and parity (2.07 ± 1.68)] and for 2nd affiliated hospital was [age (30.04 ± 4.29), gravidity (2.19 ± 1.38) and parity (0.45 ± 0.55)]. Prevalence of top five pregnancy complications in the Chinese group was gestational diabetes mellitus at 18.41%, hypothyroidism at 15.91%, oligohydramnios at 14.39%, premature rupture of membranes at 12.17%, and anemia at 5.73%. The prevalence of the top five pregnancy complications in the Zambian group was preeclampsia at 13.80%, PIH at 12.74%, PROM at 12.45%, eclampsia at 7.53%, and placenta abruption at 7.43%. Statistical significance findings were noted as follows: Oligohydramnios [OR 0.02, CI (0.01 - 0.05), p = 0.000], placenta praevia [OR 0.08, CI (0.01 - 0.61), p = 0.015], preeclampsia [OR 13.10, CI (7.22 - 23.78), p = 0.000], placenta abruptio [OR 79.73, CI (11.07 - 574.38), p = 0.000], PIH [OR 11.95, CI (6.57 - 21.73), p = 0.005], eclampsia [OR 162.90, CI (10.08 - 2631, p = 0.000), PPROM [OR 0.03, CI (0.00 - 0.45), p = 0.012], GDM [OR 0.11, CI (0.07 - 0.17), p = 0.000], hypothyroidism [OR 0.01(0.00-0.03), p = 0.000], anemia [OR 0.18, CI (0.92-0.34), p = 0.000], ICP [OR 0.03, CI (0.00 - 0.48), p = 0.013], syphilis [OR 7.17, CI (2.14 - 24.02), p = 0.001], UTI [OR 22.55, CI (3.04 - 17.26), p = 0.002], HBV [OR 0.05, CI (0.00 - 0.86), p = 0.039] and GBS [OR 0.06, CI (0.00 - 1.11), p = 0.059].
Conclusion: Highest odds for obstetrical and infection-related pregnancy complications were associated with Zambian cases. The highest odds for medical complications were associated with Chinese cases.
Various complications occur in pregnancy ranging from mild to life-threatening diseases. These complications can range from mild to severe, sometimes life-threatening illnesses. The complications can be caused by or can be made worse by being pregnant. Some of the common complications include cardiovascular diseases, infections, hypertensive disorders, preterm labor, gestational diabetes, hyperemesis gravidarum, anemia, thromboembolism, depression and anxiety disorders [1,2].
Standard maternal health care is important for maternal and newborn health, so as the health status of that nation [3]. Several countries consider maternal and child health as an important indicator to measure the development of that country [4]. In 2000, 189 countries signed a Millennium Declaration committing them to achieve eight-millennium development goals. Millennium Development Goal number 5 was to reduce maternal mortality ratio by 75% between 1990 and 2015 [5].
Research on common medical and surgical complications has been done for women in mainland China of which PROM and anemia had the highest incidence among the obstetric and medical complications respectively [6-8]. Research done at UTH revealed that the commonest causes of maternal deaths were preeclampsia and eclampsia, septicemia, hemorrhage, and ruptured uterus [9].
In the past 10 years, there has been more reduction in child mortality than in neonatal mortality [8]. In children below 5 years, newborn mortality accounts for over 40%. About 2.6 million stillbirths happen worldwide per year, of which over 40% are pregnancy related [8,10].
Prevention of pregnancy-related perinatal mortalities requires a comprehensive multisectoral approach to quality antenatal care. The identification and proper management of maternal complications can improve maternal and perinatal outcomes [11].
Our study aims to evaluate the prevalence of pregnancy complications among racially and ethnically different women admitted at the two referral hospitals. This study may highlight the need for prevention of pregnancy complications; provide a reliable basis for health administrative departments to devise policies to control obstetrical and neonatal diseases and allow better allocation of medical resources.
Subjects
This was a two-center, cross-sectional observational study of 2030 obstetrical cases. 994 and 1036 obstetrical cases were randomly selected from the 2nd affiliated hospital of Nanjing medical university and Mansa general hospital respectively during the period January 1, 2021, to December 31, 2021. The selection was done by probability sampling methods which included simple random sampling and systematic sampling methods. Our study included all patients admitted to the hospital's obstetrics ward. Only pregnant patients with newly diagnosed complications were recruited in the study regardless of their age, gestational age, or site of pregnancy. Information about the cases was electronically and manually recorded, including general conditions, medical history, pregnancy history, and complications.
Data extraction
Data was collected from the hard copy files and electronic inpatients’ medical records. These records had all the information required for the study including patient history, physical examination, investigations, and relevant management. For analysis purposes, pregnancy complications were categorized into infection related, obstetric, and medical.
Diagnosis of the complications
This was performed in accordance with the standards in the 24th Edition of the Williams Obstetrics textbook.
Statistical analysis
Statistical analysis was performed using SPSS software version 23. Continuous variables were summarized using descriptive statistics, including the number of subjects, mean, standard deviation, median, and confidence intervals with minimum and maximum values. Demographic characteristics were expressed as numbers and frequency distributions for categorical variables. Maternal characteristics were evaluated using the two samples t-test. The odds ratios and confidence intervals were calculated as measures of association between ethnicity/race using a binary logistic regression model. Confidence interval was set at 95% and p < 0.05 (2-tailed) was considered statistically significant.
Ethics statement: The study was approved by the Hospital research committee of the second affiliated hospital of Nanjing Medical University and Mansa general hospital.
758/994 (76.25%) Chinese and 703/1036 (67.86%) Zambian sampled patients were affected by one or more pregnancy complications.
Subjects characteristics
The summary of patient characteristics is shown in Table 1.
| Table 1: Maternal characteristics. | |||||||
| Characteristic | Total n (%) | Mean ± SD | t | 95% CI | P value | ||
| 2nd affiliated hospital of NMU | Mansa general hospital | 2nd affiliated hospital of NMU | Mansa general hospital | ||||
| Total patients
AGE (years) <18 18 - 25 26 - 30 31 - 35 >35 |
994 (100%)
3 (0.30) 133 (13.38) 426 (42.35) 323 (32.49) 114 (11.47) |
1036(100%)
63 (6.08) 467 (45.08) 177 (17.08) 170 (16.41) 159 (15.35) |
30.04 ± 4.29 | 26.69 ± 7.33 | -12.60 | -3.90-(-2.85) | 0.000 |
| GRAVIDITY Gravida 1 Gravida 2 Gravida 3 Gravida >3 |
396 (39.84) 283 (28.47) 150 (15.09) 165 (16.60) |
255 (24.61) 155 (14.96) 210 (20.27) 416 (40.15) |
2.19 ± 1.38 | 3.35 ± 2.08 | 14.72 | 1.00 - 1.31 | 0.000 |
| PARITY Para 0 Para 1 Parity >1 |
578 (58.15) 390 (39.2) 26 (2.62) |
255 (24.61) 155 (14.96) 627 (60.42) |
0.45 ± 0.55 | 2.07 ± 1.68 | 29.12 | 1.52 - 1.74 | 0.000 |
Maternal age: Age records for all the admitted pregnant women were documented. The mean age was 30.04 and 26.69 years in Chinese and Zambians respectively. Our research reviewed that the peak delivery ages for Zambian cases were younger (18 - 25 years old, 467/1036 cases 45.08%) as compared to Chinese cases (26 - 30 years old, 42.35%). The youngest and oldest admitted cases were found to be higher among the Zambian women (<18 years, 63/1036 cases 6.08% and > 35 years, 159/1036 cases 15.35%) as compared to Chinese women (<18 years, 3/994 cases 0.30% and >35 years, 114/994 cases 11.47%). Overall, there was a significant difference in mean age between the Zambians and the Chinese women (p = 0.000).
Gravidity: The mean gravidity was 2.19 and 3.35 in Chinese and Zambian women respectively. Patients with gravidity of ≥3 were higher in Zambian cases than in Chinese cases. There was a significant difference in mean gravidity between the Zambians and the Chinese women (p = 0.000).
Parity: The mean parity was 0.45 and 2.07 among Chinese and Zambian cases respectively. 627 cases (60.42%) have had a parity of >1 in Zambian cases compared to Chinese women (26/994 cases 2.62%). Maximum parity was 8 (in Zambian cases) and 3 (in Chinese cases). There was a significant difference in mean parity between the Zambians and the Chinese women (p = 0.000).
Pregnancy complications
Pregnancy complications were grouped into obstetrical, medical, and infection-related [Table 2].
| Table 2: Frequencies and proportions of maternal complications by hospital. | ||||
| Complication | n (%) | OR (CI) | p value | |
| Mansa general hospital | 2nd affiliated hospital of NMU | |||
| Total patient’s N (100%)
Obstetrical complications: Oligohydramnios Premature rupture of membranes Preterm labor Placenta praevia Polyhydramnios Abortions Preeclampsia Placenta abruption Pregnancy-induced hypertension Eclampsia PPROM Medical complications: Gestational diabetes mellitus Hypothyroidism Anemia ICP Infection-related complications: Vaginal candidiasis Syphilis Urinary tract infection Viral hepatitis B Group B streptococcal infection chorioamnionitis |
1036 (100%)
3 (0.29) 121 (12.45) 52 (5.02) 1 (0.10) 12 (1.16) 1 (0.10) 143 (13.80) 77 (7.43) 132 (12.74) 78 (7.53) 0 (0.00) 25 (2.41) 1 (0.10) 11 (1.06) 0 (0.00) 1 (0.10) 22 (2.12) 23 (2.22) 0 (0.00) 0 (0.00) 0 (0.00) |
994 (100%)
143(14.39) 121(12.17) 56 (5.63) 12 (1.21) 6 (0.60) 6 (0.60) 12 (1.21) 1 (0.10) 12 (1.21) 0 (0.00) 17 (1.71) 183(18.41) 150(15.91) 57 (5.73) 16 (1.61) 2 (0.20) 3 (0.30) 1 (0.10) 9 (0.91) 7 (0.70) 1 (0.10) |
0.02(0.01 - 0.05) 0.96(0.73 -1.25) 0.89(0.60 - 1.30) 0.08(0.01 - 0.61) 1.93(0.72 - 5.16) 0.16(0.02 - 1.32) 13.10(7.22 - 23.78) 79.73(11.07 - 574.38) 11.95(6.57 - 21.73) 162.90(10.08 - 2631) 0.03(0.00 - 0.45) 0.11(0.07 - 0.17) 0.01(0.00 - 0.03) 0.18(0.92 - 0.34) 0.03(0.00 - 0.48) 0.48(0.04 - 5.29) 7.17(2.14 - 24.02) 22.55(3.04 - 17.26) 0.05(0.00 - 0.86) 0.06(0.00 - 1.11) 0.30(0.01 - 7.46) |
0.000 0.732 0.538 0.015 0.190 0.089 0.000 0.000 0.005 0.000 0.012 0.000 0.000 0.000 0.013 0.548 0.001 0.002 0.039 0.059 0.465 |
| OR = 0 no outcome event for the ethnicity OR = 1 exposure does not affect the odds of the outcome OR > 1 exposure associated with higher odds of the outcome OR < 1 exposure associated with lower odds of the outcome The covariates included in the logistic regression model were ethnicity. |
||||
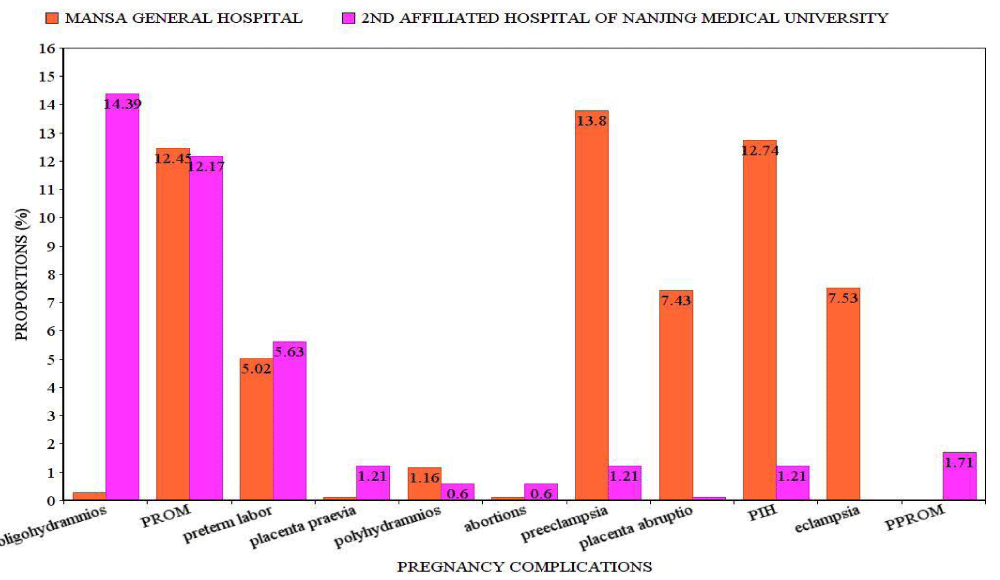
Obstetrical complications: Statistical significance findings were seen (p = 0.000) among the two ethnic groups with oligohydramnios (p = 0.000), placenta praevia (p = 0.015), Preeclampsia (p = 0.000), Placenta abruption (p = 0.000), Pregnancy induced hypertension (p = 0.005), Eclampsia (p = 0.000) and PPROM (p = 0.012) (Table 2). The odds of developing oligohydramnios, placenta praevia, and preterm premature rupture of membranes were higher in Chinese women than in Zambian women. No significant findings were seen in premature rupture of membranes, preterm labor, polyhydramnios, and abortions between the Zambians and the Chinese women. In Chinese women, there was an increased proportion of oligohydramnios (143/994 cases, 14.39%), preterm labor (56/994 cases, 5.63%), placenta praevia (12/994 cases, 1.21%) and abortions (6/994 cases 0.60%) than in Zambians. Preeclampsia (143/1036 cases 13.80%), placenta abruption (77/1036 cases 7.43%), pregnancy-induced hypertension (132/1036 cases, 12.74%), premature rupture of membranes (121/1036 cases 12.45%) and eclampsia (78/1036 cases, 7.53%) were higher in Zambian than in Chinese cases. The highest proportion was oligohydramnios (143/994 cases, 14.39%) among the Chinese patients, while for Zambian patients it was preeclampsia (143/1036 cases, 13.80%) (Figure 1).
Figure 1: Proportions of obstetrical complications. PROM: Premature Rupture of Membranes; PIH: Pregnancy-Induced Hypertension; PPROM: Preterm Premature Rupture of Membranes.
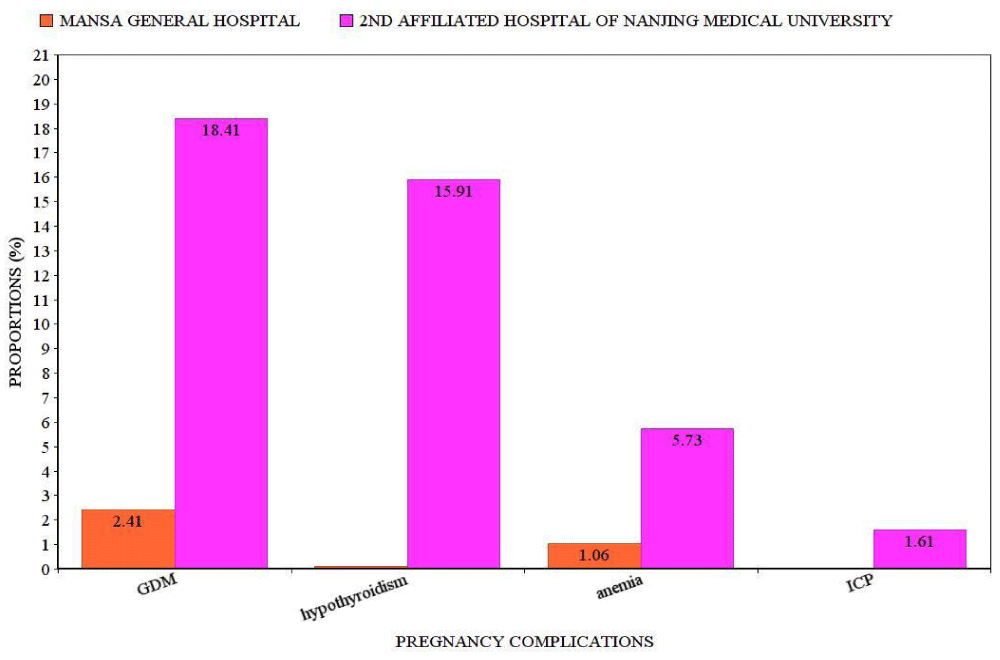
Medical: In this study, a high proportion of cases were found in the Chinese than in Zambians. Gestational diabetes mellitus (183/994 cases, 18.41%) had the highest proportion in this category and our overall study (Figure 2). Gestational diabetes mellitus, hypothyroidism, anemia and ICP had increased significantly (p = 0.000). The odds of having Gestational diabetes mellitus, hypothyroidism, anemia, and ICP was higher in Chinese women than in Zambian cases (Table 2).
Figure 2: Proportions of medication complications. GDM: Gestation Diabetes Mellitus; ICP: Intrahepatic Cholestasis of Pregnancy.
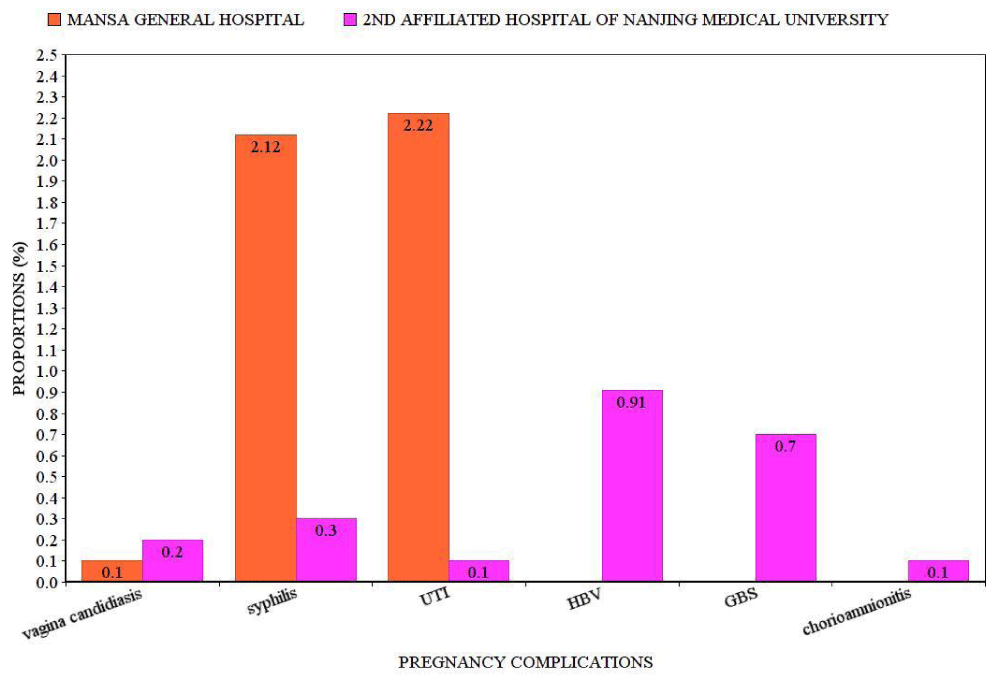
Infection-related complications: High proportions of urinary tract infections were noted among Zambians (23/1036 cases, 2.22%) than Chinese (1/994 cases. 0.10%) patients (Figure 3). Syphilis was higher in Zambians (22/1036 cases, 2.12%) than in Chinese (3/994 cases 0.30%). A significant increase in syphilis (p = 0.001) and urinary tract infection (p = 0.002) among the Zambian cases was seen. A significant increase in hepatitis B virus (p = 0.039) and group B streptococcal infection (p = 0.059) was seen. There was no statistical significance in vagina candidiasis and chorioamnionitis between the groups (Table 2).
Figure 3: Proportions of infection related complications.UTI:Urinary Tract Infection; HBV:Hepatitis B Virus; GBS:Group B Streptococcal Infection.
The determinants of population health status and the primary explanations of disparities among population groups lie in the social, physical, and economic environments, which in turn are determined by the larger society's norms, values, social stratification systems, and political economy (King, 1996; Menefee, 1996). Black/white disparities in health status have consistently been attributed to such variables as socioeconomic status (especially income, lack of education, and unemployment); lifestyle choices and behavioral risks; occupational and environmental hazards, inferior housing, poor nutrition, and different cultural beliefs about health and illness. There is evidence for all of these variables. Another explanation is the lack of black people's access to health care, particularly the lack of either public or private health insurance (Blendon, et al. 1989; Weinick, Zuvekas, and Cohen, 2000).
The common possible reasons for racial and ethnic disparities are Cultural and language barriers; Time limitations imposed by the pressures of clinical practice; Distrust by many minority patients; A woeful lack of minority physicians who may be more culturally sensitized to the needs of their patients; Conscious or subconscious biases, prejudices, and negative racial stereotypes or perceptions that affect the way providers deliver care.
To address, advance action, and promote change the following can be done: Raise the visibility of racial and ethnic health disparities as a national problem; Promote the development of programs and strategies to reduce disparities; Foster leadership to effect change; and Track promising activities and developments in minority health care that could lead to dramatically reducing or eliminating disparities.
To the best of our knowledge, this is the first study comparing the common pregnancy complications between racially and ethnically different Chinese and Zambian gravid women.
Subject characteristics
Maternal age: Women aged 26 years – 30 years (426/994 cases, 42.86%) formed the majority of admissions for Chinese patients. The findings are consistent with the childbearing age of China which is 27.4 years [12]. Some of the reasons for increased pregnancies among this age group are financial stability, improved mental strength, and family pressure to get married and have children, and by then, many could have attended tertiary education. The proportion of younger teenage (< 18 years) pregnancies was 0.30% (3/994 cases) as compared to the Chinese adolescent rate of 3.4% in 2019 [13]. An increase in the number of teenage pregnancies has been noted among Chinese women. This may be due to early marriage, poverty, or inadequate use of contraceptives. Chinese people live in a conservative society where sexual health discussions are minimal leading to inadequate sexual education which eventually can lead to low levels of sexual knowledge, unprotected sexual intercourse, and unwanted pregnancies. Women who were >35 years accounted for 11.47% of the admissions similar to the findings of the multicenter Chinese study done in 2011 (10.05%) [14]. Many maternal, fetal, and neonatal bad outcomes have been associated with advanced maternal age [15]. For Zambians admitted at Mansa general hospital, the majority of cases were 18 years - 25 years (467/1036 cases, 45.08%). This hospital is located in the rural province of Zambia called Luapula. Initial sexual intercourse median age for women aged 25-49 is 16.6 years, compared with 18.5 years for men aged 25 - 49. Those women who are educated begin sexual activity 4.2 years later than women who are not educated (20.0 years vs. 15.8 years). About 17 percent of women begin sexual activity before they attain 15 years of age; while 69% begin sexual activity before they reach the age of 18. Men marry later compared to women. 34% of women have had a child by age 18 [16]. In many rural parts of Zambia, many teenagers commence sexual activities due to culture, lack of education, poverty, and family pressure [17].
Gravidity: Gravida ≤2 patients were high in Chinese than in Zambian cases. The one-child policy which was introduced by the Chinese government is still being practiced by many Chinese families; hence the low number of pregnancies. Other reasons include long working hours with less family time and the notion of having many children leading to more expenses. For Zambian women, cases with gravida ≥ 3 were higher than for the Chinese. In Zambia, especially in rural areas, some cultures allow men to marry more than one wife, there is no limitation on the number of children a couple may have. Rooted traditions are still active and well-anchored, with poor access to contraception [18].
Parity: Parity ≤1 was predominant among Chinese patients while those with parity >1 were high in Zambian patients. The steady rise in parity among women in China has been noted. According to the 2020 Chinese census, the number of babies born was less than those in 2019. This led the government to permit couples to have three children [19]. High parity is a major health concern among developing countries, especially in rural areas where MGH is located. High parity is a risk factor for several maternal complications and neonatal outcomes [20]. Some factors may influence the parity and health status of women. These include poverty, level of education, early marriages, unemployment, and cultural factors [21].
Previous abortions: The number of abortions was significantly higher among Chinese (0.60%) than in Zambian (0.10%) cases (Table 2). Abortions occur at a rate of 37 abortions per 1000 women in poor countries for women aged 15 years - 44 years [22]. In Zambian, it is legal to abort to protect the health status of a pregnant woman or any of her children [9]. Abortions are only provided in registered hospitals, approved by three medical doctors of whom one must be a specialist obstetrician [9]. This process hinders women to opt for abortion on most occasions. Induced abortion as a mode of family planning in China is widely used. Official data in 2019 reviewed about 9.76 million abortions of which 50% were repeated [23,24].
Pregnancy complications
758/994 (76.26%) sampled Chinese and 703/1036 (67.86%) Zambian patients were affected by one or more pregnancy complications.
a) Medical complications
Gestational diabetes mellitus: Prevalence worldwide is 1-45% of pregnancies [25,26]. According to a study done in 2019, the total incidence of GDM in mainland China was 14.8% (95% CI 12.8% - 16.7%) [27]. The incidence in our study was 18.41% (183/994 cases) and there was a significant association of GDM with the Chinese than Zambian cases. Various ethnicities and genetic makeup have different rates of gestational diabetes mellitus [28-31]. Many differences in the diagnosis of GDM result in variations in incidences [32-34].
Data sources used in the calculation of prevalence may have an impact on the reporting of GDM [35,36]. Inadequate case findings and lack of equipment to use for the diagnosis of GDM among Zambian cases can be one of the factors affecting the results in this group.
Hypothyroidism: Prevalence in Asia than Western countries. If left untreated, it can increase the risk of various pregnancy ailments [37]. Prevalence in pregnancy is about 2.5% according to the Western literature [38]. In our study, the prevalence was 15.91% in Chinese patients, higher than the global prevalence; the prevalence in Zambian patients was 0.10%. The odds were higher in Chinese than in Zambian cases. The reason for this variance may be due to the prioritized screening of hypothyroidism to all pregnant women admitted to our hospital and also the ethnicity of the women under study. No collective agreement has been made universally to screen and treat this complication in pregnancy.
According to the American College of Obstetricians and Gynecologists, examination of thyroid function is only done in women with symptoms of thyroid disease, women with a history of thyroid disease, and other associated diseases [37,39]. The size of the thyroid gland increase in pregnancy by 10% in countries with sufficient iodine than in iodine deficiency countries [40]. Physiologically, the thyroid hormone production and iodine requirement all increase by about 50% during pregnancy [41]. Stressful events for the thyroid gland lead to hypothyroidism in women with inadequate thyroid reserve or iodine.
Anemia: The global prevalence of anemia for women of reproductive age was 29.4%, 38.2% in pregnant women, and 29.0% in non-pregnant women [42]. In China, the prevalence of anemia in pregnancy was 19.8% [43]; the World Bank puts the prevalence of anemia in pregnancy in Zambia at 39.1% [44]. In our study, the prevalence of anemia among Chinese was 5.73% (57/994 cases) and 1.06% (11/1036 cases) in Zambian cases. Our prevalence was lower than the global prevalence. The odds of developing anemia were higher in Chinese than in Zambian cases. Reasons for the differences could be due to the socio-demographic characteristics of pregnant women [45], variations across geographic regions in China and other countries which might be due to differences in the kind of food eaten and cultural beliefs about the food to eat during pregnancy [46].
Intrahepatic cholestasis of pregnancy: Disorder with features of itching, raised serum liver enzymes and bile acid levels, onset in the second or third trimester, and spontaneous relief of signs and symptoms within two to three weeks after delivery [47,48]. Globally it ranges from < 1 to 27.6% [49,50]. It is common in South Asia, South America, and Scandinavia people. The cause of ICP during pregnancy is due to many factors. It may be associated with raised estrogen levels and compromised hepatobiliary transport proteins during pregnancy [51,52]. Studies have shown its association with spontaneous and iatrogenic preterm delivery [53-55]. Chinese prevalence in our research was 1.61% (16/994 cases) within the global range. The odds of developing anemia were higher in Chinese than in Zambian cases.
b) Obstetric complications
Oligohydramnios: A condition is commonly seen after the first trimester with an Amniotic Fluid Index (AFI) less than 5 cm [24]. In a study done in 2011 involving 39 Chinese hospitals, the incidence of oligohydramnios was found to be 4.4% [25]. In our current study, oligohydramnios incidence was 14.39% (143/994 cases) in Chinese patients, higher than the global incidence which is 1% - 5% of pregnancies at term [26]. Zambian patients had an incidence of 0.29%. This difference may be related to the different diagnostic accuracy modalities in various regions of mainland China and the world. Another reason may be the increased surveillance with the use of ultrasound to diagnose this condition among Chinese patients. The odds of developing oligohydramnios were higher in Chinese than in Zambian cases.
Premature rupture of membranes (PROM): Globally, the prevalence of PROM IS 5% – 10% [59]. It is associated with poor perinatal and neonatal outcomes [60]. Based on the current study, PROM accounted for 12.45% (121/1036 cases) in Zambian and 12.17% (103/994 cases) in Chinese cases. These rates are similar to the global trend. PROM significantly increases the chances of developing intrauterine infection (chorioamnionitis) [61]. Early identification and management of bacterial vaginosis and E. coli infection in pregnancy may reduce the risk of PROM [62]. Overall, no statistical significance was found in our study between the groups (p = 0.732).
Preterm labor: Preterm births range from 5% in developed countries to 25% in poor countries [63]. In our study, the incidence was found to be 5.63% (56/994 cases) among Chinese higher than 5.02% (52/1036 cases) in Zambian patients. These rates are similar to the range of developed countries which has been stable at 5% - 10% for many years. Our findings differ from other research findings which have shown black women have increased rates of preterm births [63]. Ethnic differences can only explain a very small proportion of global preterm births. Some factors associated with preterm labor include infections, hypertension, preeclampsia, IUGR, and heavy physical work [63]. There was no statistical significance found in our study between the groups (p = 0.538).
Preeclampsia: 2% - 8% of pregnancies worldwide are affected by this condition [64]. 9% of maternal deaths in developing countries in Africa and Asia are associated with preeclampsia [65]. In Zambia, Lusaka, prevalence is at 18.9% [66] higher than our findings of 13.80% among the Zambian cases in the Mansa district. Research in 2021 showed that the prevalence of preeclampsia was 2.2% in Chinese women [67], similar to our research findings of 1.21% in Chinese patients. In our study, the odds were high in Zambian than in Chinese cases. Several biophysical and demographic factors are associated with preeclampsia. Some of the risk factors include chronic hypertension, chronic kidney disease, insulin-dependent diabetes, and previous preeclampsia. Other factors comprise in vitro fertilization, family history of preeclampsia, advanced maternal age, obesity, multiple pregnancy, and nulliparity [68-70]. Research has revealed that black women have an increased risk of developing preeclampsia [70]. Poor maternal socioeconomic status increases the risk of preeclampsia [71]. A study in Lusaka, Zambia, has reviewed that low level of education, previous history of preeclampsia in the previous pregnancy, being single, divorced or widowed, and having parity of three more were attributed to preeclampsia. Daily vegetable intake, fruit intake, and nutritional counseling during antenatal care have been shown to be protective modalities against preeclampsia [72].
PIH: About 6% - 10% of pregnancies are affected [73]. It is defined as raised Systolic Blood Pressure (SBP) >140 mmHg and diastolic blood pressure (DBP) >90 mmHg. It is classified into mild, moderate, and severe [74]. Women with hypertension, collagen vascular disease, obesity, black race, insulin resistance, diabetes mellitus, gestational diabetes, increased serum testosterone concentrations, and thrombophilia are at risk of developing PIH [74,75]. Our research showed a higher proportion among black Zambians (12.74%) above global rates than in Chinese (1.21%). Odds were higher among Zambians than in Chinese cases. Studies have shown that black women have a high risk of hypertensive disorders in pregnancy while Asian women have a low risk [76]. It has been hypothesized that the causes of hypertensive disease in pregnancy HDP could be due to problems of placental implantation and the level of trophoblastic invasion [77]. Several studies worldwide have been done showing variations among people with different geographical and ethnicities [78]
Eclampsia: Globally, the rate range from 1 case per 100 pregnancies to 1 case per 1700 pregnancies [79]. It has a high burden on maternal and neonatal mortality and morbidity. The prevalence of eclampsia among the Chinese was higher than among Zambians for our study. According to a survey done in China on the six subtypes of hypertensive disease in pregnancy, eclampsia was at a rate of 0.89% [80] similar to our research findings. Eclampsia is one of the worst outcomes of hypertensive diseases in pregnancy because of its unpredictable evolution as well as its potentially severe complications. Many pregnant women die from preeclampsia due to eclampsia [81]. Numerous improvements in the management of the disease have been made but, we still have high rates of maternal and perinatal mortality [82-84]. The frequency of eclampsia varies with the level of development across different countries. This frequency is linked to the quality of antenatal care. Well-organized health systems are able to significantly reduce this complication of preeclampsia, given that precursor signs tend to occur much earlier in pregnancy. Odds were higher among Zambians than in Chinese cases.
Placenta abruption: Complete or partial separation of a normally implanted placenta before delivery. The cause is multifactorial and its etiopathogenetic mechanism is not yet entirely understood [85]. Incidence in developing countries ranges from 4% - 6% with increased risk of maternal and fetal morbidity and mortality [86,87]. Many risk factors have been attributed to placenta abruption. Some examples are previous history of placenta abruption, advanced maternal age, previous cesarean section, grand multiparity, twin pregnancies, diabetes mellitus, cigarette smoking, chronic hypertension, preeclampsia, premature rupture of membranes, abdominal trauma, and polyhydramnios [88-90].
In our study, the prevalence among Zambians (7.43%) was higher than in the Chinese patients (0.10%). The 0.10% is similar to the study done in Hebei province of China (0.31%) [91], and lower than the global range. The rate in the Zambian patients (7.42%) was higher than the global range. Similar to our research, some studies have shown that black race is a risk factor for placenta abruption. Studies have shown that black births were complicated by placental abruption, compared to White births [92]. In our study, the odds were higher among Zambians than in Chinese cases.
c) Infection related
82.5% of UTIs are caused by Escherichia coli [93]. Other bacteria which may be seen are Klebsiella pneumoniae, Staphylococcus, Streptococcus, Proteus, and Enterococcus species. A high proportion (2.22%) of urinary tract infections was seen among Zambians than among Chinese (0.10%). Findings for MGH were lower than the research findings done in Zambia involving 203 cases with a UTI prevalence of 60% (95% CI: 53.3% – 66.7%) [94]. Factors influencing the development of UTIs are immunological changes during pregnancy, changes in the urinary tract by ureter and renal calyces dilatation, reduced bladder capacity, and vesicoureteral reflux all due to progesterone-related smooth muscle relaxation. Other factors are low socioeconomic status, anemia, and sexual activity [95].
Our study showed limitations. A significant proportion of disease data were missing or not recorded due to differences in the criterion by which diagnoses were arrived at between the Mansa general hospital and the second affiliated hospital of Nanjing medical university.
For obstetric complications, hypertensive disorders of pregnancy and placenta abruption were significantly high in Zambian women. While oligohydramnios, placenta praevia, and PROM were significantly high in Chinese patients. Medical conditions which include GDM, hypothyroidism, anemia, and intrahepatic cholestasis of pregnancy were significantly high in Chinese patients. Infections namely syphilis and UTIs were high among Zambian women why viral hepatitis was common among the Chinese, Surveillance, early identification and management of these pregnancy complications are very paramount in ensuring the safety of the mother and the fetus. This could yield benefits for improving maternal outcomes, but could also reduce perinatal mortality rates. A comprehensive antenatal care approach is critical in identifying these risk pregnancies. Furthermore, future reassert maybe be required to study the genetic differences between black women and white women.
Ethics approval and consent to participate
The study and raw patient data access were approved by the hospital research committees. Informed consent for patients was waived because of the retrospective nature of the study. The data used in the study was anonymized before its use.
Availability of data and materials
Data presented in this study can be provided upon request from the corresponding author. Due to public restrictions, data is not publicly available.
Competing interests: There are no conflicts of interest regarding the publication of this paper
Author contribution
Concept, design, and data analysis with interpretation were done by KC and YXY. The original draft of the manuscript was done by KC. WY, LSL, KYY, HMY, GYS, and HQ were involved in the interpretation of clinical data from the Chinese language to the English language. RNN was responsible for the collection and organization of patient data for Mansa general hospital cases. After the review of the manuscript content by all authors, it was agreed that the final version be submitted for publication.
We are thankful to all the doctors and nurses in the Department of Obstetrics and Gynecology at the second affiliated hospital of Nanjing Medical University and Dr. Royd Nkalamo Nonde of Mansa general hospital for the support rendered in this study.
- World Health Organization. Maternal mortality 2019. Retrieved. January 15,2020, from https://www.who.int/en/news-room/fact-sheets/detail/maternal-mortality
- CDC. Pregnancy complications 2018. Retrieved March 3, 2020, from https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications.html.
- Lassi ZS, Kedzior SG, Bhutta ZA. Community-based maternal and newborn educational care packages for improving neonatal health and survival in low- and middle-income countries. Cochrane Database Syst Rev. 2019 Nov 5;2019(11):CD007647. doi: 10.1002/14651858.CD007647.pub2. PMID: 31686427; PMCID: PMC6828589.
- Blencowe H, Krasevec J, de Onis M, Black RE, An X, Stevens GA, Borghi E, Hayashi C, Estevez D, Cegolon L, Shiekh S, Ponce Hardy V, Lawn JE, Cousens S. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2019 Jul;7(7):e849-e860. doi: 10.1016/S2214-109X(18)30565-5. Epub 2019 May 15. PMID: 31103470; PMCID: PMC6560046.
- Liang J, Li X, Kang C, Wang Y, Kulikoff XR, Coates MM, Ng M, Luo S, Mu Y, Wang X, Zhou R, Liu X, Zhang Y, Zhou Y, Zhou M, Li Q, Liu Z, Dai L, Li M, Zhang Y, Deng K, Zeng X, Deng C, Yi L, Zhu J, Murray CJL, Wang H. Maternal mortality ratios in 2852 Chinese counties, 1996-2015, and achievement of Millennium Development Goal 5 in China: a subnational analysis of the Global Burden of Disease Study 2016. Lancet. 2019 Jan 19;393(10168):241-252. doi: 10.1016/S0140-6736(18)31712-4. Epub 2018 Dec 13. PMID: 30554785; PMCID: PMC6336935.
- Luo XL, Zhang WY. Obstetrical disease spectrum in China: an epidemiological study of 111,767 cases in 2011. Chin Med J (Engl). 2015 May 5;128(9):1137-46. doi: 10.4103/0366-6999.156076. PMID: 25947393; PMCID: PMC4831537.
- Luo XL, Zhang WY. Obstetrical disease spectrum in China: an epidemiological study of 111,767 cases in 2011. Chin Med J (Engl). 2015 May 5;128(9):1137-46. doi: 10.4103/0366-6999.156076. PMID: 25947393; PMCID: PMC4831537.
- Lawn JE, Kinney MV, Black RE, Pitt C, Cousens S, Kerber K, Corbett E, Moran AC, Morrissey CS, Oestergaard MZ. Newborn survival: a multi-country analysis of a decade of change. Health Policy Plan. 2012 Jul;27 Suppl 3:iii6-28. doi: 10.1093/heapol/czs053. Erratum in: Health Policy Plan. 2013 Oct;28(7):786-8. PMID: 22692417.
- Hickey MU, Kasonde JM. Maternal mortality at University Teaching Hospital, Lusaka. Med J Zambia. 1977 Jun-Jul;11(3):74-8. PMID: 302537.
- McClure EM, Wright LL, Goldenberg RL, Goudar SS, Parida SN, Jehan I, Tshefu A, Chomba E, Althabe F, Garces A, Harris H, Derman RJ, Panigrahi P, Engmann C, Buekens P, Hambidge M, Carlo WA; NICHD FIRST BREATH Study Group. The global network: a prospective study of stillbirths in developing countries. Am J Obstet Gynecol. 2007 Sep;197(3):247.e1-5. doi: 10.1016/j.ajog.2007.07.004. PMID: 17826406; PMCID: PMC2150563.
- Vogel JP, Souza JP, Mori R, Morisaki N, Lumbiganon P, Laopaiboon M, Ortiz-Panozo E, Hernandez B, Pérez-Cuevas R, Roy M, Mittal S, Cecatti JG, Tunçalp Ö, Gülmezoglu AM; WHO Multicountry Survey on Maternal and Newborn Health Research Network. Maternal complications and perinatal mortality: findings of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014 Mar;121 Suppl 1:76-88. doi: 10.1111/1471-0528.12633. Erratum in: BJOG. 2015 Feb;122(3):451. PMID: 24641538.
- Powell Bill. Gender Imbalance: How China's One-Child Law Backfired on Men; China's one-child policy has had one unlikely aftereffect: empowering women. Newsweek, 5 June 2015. AcademicOneFile, http://link.galegroup.com/apps/doc/A415402385/AONE?u=cuny_guttman&sid=AONE&xid=d6f643f6. Accessed 14 May 2018.
- Guo C, Pang L, Ding R, Song X, Chen G, Zheng X. Unmarried Youth Pregnancy, Outcomes, and Social Factors in China: Findings From a Nationwide Population-Based Survey. Sex Med. 2019 Dec;7(4):396-402. doi: 10.1016/j.esxm.2019.07.002. Epub 2019 Aug 23. PMID: 31451396; PMCID: PMC6963114.
- Luo XL, Zhang WY. Obstetrical disease spectrum in China: an epidemiological study of 111,767 cases in 2011. Chin Med J (Engl). 2015 May 5;128(9):1137-46. doi: 10.4103/0366-6999.156076. PMID: 25947393; PMCID: PMC4831537.
- Dekker R, Breakey AA. Evidence on advanced maternal age. Evidence Based Birth. 2016.
- Zambia Demographic and Health Survey 2018.
- Population Council, UNFPA, and Government of the Republic of Zambia. 2017. “Adolescent Pregnancy in Zambia.” Lusaka, Zambia.
- Ashraf N, Field E, Rusconi G, Voena A, Ziparo R. Traditional Beliefs and Learning about Maternal Risk in Zambia. Am Econ Rev. 2017 May;107(5):511-5. doi: 10.1257/aer.p20171106. PMID: 29553631.
- China allows three children in major policy shift. 2021. https://www.bbc.com/news/world-asia-china-57303592
- Al-Shaikh GK, Ibrahim GH, Fayed AA, Al-Mandeel H. Grand multiparity and the possible risk of adverse maternal and neonatal outcomes: a dilemma to be deciphered. BMC Pregnancy Childbirth. 2017 Sep 19;17(1):310. doi: 10.1186/s12884-017-1508-0. PMID: 28927391; PMCID: PMC5606064.
- Ngoma CM. Factors Influencing Women’s Optimum Health in Zambia. J Healthc Commun. 2016; 1:4. DOI: 10.4172/2472-1654.100030
- Sedgh G, Bearak J, Singh S, Bankole A, Popinchalk A, Ganatra B, Rossier C, Gerdts C, Tunçalp Ö, Johnson BR Jr, Johnston HB, Alkema L. Abortion incidence between 1990 and 2014: global, regional, and subregional levels and trends. Lancet. 2016 Jul 16;388(10041):258-67. doi: 10.1016/S0140-6736(16)30380-4. Epub 2016 May 11. PMID: 27179755; PMCID: PMC5498988.
- China NHCO.Health Statistics Yearbook of China. Peking Union Medical College Press; Beijing, China: 2019.
- Zhang W, Che Y. Intervention Study of Post-Abortion Family Planning services in China: Design and Implementation of the EU-PF7 INPAC Project. China Population Publishing House; Beijing, China: 2017.
- Agarwal MM, Dhatt GS, Othman Y. Gestational diabetes: differences between the current international diagnostic criteria and implications of switching to IADPSG. J Diabetes Complications. 2015 May-Jun;29(4):544-9. doi: 10.1016/j.jdiacomp.2015.03.006. Epub 2015 Mar 19. PMID: 25837380.
- Buckley BS, Harreiter J, Damm P, Corcoy R, Chico A, Simmons D, Vellinga A, Dunne F; DALI Core Investigator Group. Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening. A review. Diabet Med. 2012 Jul;29(7):844-54. doi: 10.1111/j.1464-5491.2011.03541.x. PMID: 22150506.
- Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J Diabetes Investig. 2019 Jan;10(1):154-162. doi: 10.1111/jdi.12854. Epub 2018 May 27. PMID: 29683557; PMCID: PMC6319492.
- Pu J, Zhao B, Wang EJ, Nimbal V, Osmundson S, Kunz L, Popat RA, Chung S, Palaniappan LP. Racial/Ethnic Differences in Gestational Diabetes Prevalence and Contribution of Common Risk Factors. Paediatr Perinat Epidemiol. 2015 Sep;29(5):436-43. doi: 10.1111/ppe.12209. Epub 2015 Jul 22. PMID: 26201385; PMCID: PMC6519933.
- Savitz DA, Janevic TM, Engel SM, Kaufman JS, Herring AH. Ethnicity and gestational diabetes in New York City, 1995-2003. BJOG. 2008 Jul;115(8):969-78. doi: 10.1111/j.1471-0528.2008.01763.x. PMID: 18651880.
- Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, Stampfer MJ, Speizer FE, Spiegelman D, Manson JE. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997 Oct 1;278(13):1078-83. PMID: 9315766.
- Saker PJ, Hattersley AT, Barrow B, Hammersley MS, McLellan JA, Lo YM, Olds RJ, Gillmer MD, Holman RR, Turner RC. High prevalence of a missense mutation of the glucokinase gene in gestational diabetic patients due to a founder-effect in a local population. Diabetologia. 1996 Nov;39(11):1325-8. doi: 10.1007/s001250050577. PMID: 8932999.
- Djelmis J, Pavić M, Mulliqi Kotori V, Pavlić Renar I, Ivanisevic M, Oreskovic S. Prevalence of gestational diabetes mellitus according to IADPSG and NICE criteria. Int J Gynaecol Obstet. 2016 Dec;135(3):250-254. doi: 10.1016/j.ijgo.2016.07.005. Epub 2016 Aug 27. PMID: 27612531.
- Berggren EK, Boggess KA, Stuebe AM, Jonsson Funk M. National Diabetes Data Group vs Carpenter-Coustan criteria to diagnose gestational diabetes. Am J Obstet Gynecol. 2011 Sep;205(3):253.e1-7. doi: 10.1016/j.ajog.2011.06.026. Epub 2011 Jun 15. PMID: 22071053; PMCID: PMC3670957.
- Ekeroma AJ, Chandran GS, McCowan L, Ansell D, Eagleton C, Kenealy T. Impact of using the International Association of Diabetes and Pregnancy Study Groups criteria in South Auckland: prevalence, interventions and outcomes. Aust N Z J Obstet Gynaecol. 2015 Feb;55(1):34-41. doi: 10.1111/ajo.12267. Epub 2014 Oct 11. PMID: 25307052.
- Lawrence JM. Prevalence of GDM. In: Kim C, Ferrara A, editors. Gestational diabetes during and after pregnancy. 2010; 53–69.London: Springer.
- Pedersen ML, Olesen J, Jørgensen ME, Damm P. Gestational diabetes mellitus in Greenland: a national study of prevalence and testing efficacy. Int J Circumpolar Health. 2016 Aug 24;75:32167. doi: 10.3402/ijch.v75.32167. PMID: 27562574; PMCID: PMC4999506.
- LeBeau SO, Mandel SJ. Thyroid disorders during pregnancy. Endocrinol Metab Clin North Am. 2006 Mar;35(1):117-36, vii. doi: 10.1016/j.ecl.2005.09.009. PMID: 16310645.
- American College of Obstetrics and Gynecology. ACOG practice bulletin. Thyroid disease in pregnancy. Number 37, August 2002. American College of Obstetrics and Gynecology. Int J Gynaecol Obstet. 2002 Nov;79(2):171-80. doi: 10.1016/s0020-7292(02)00327-2. PMID: 12481755.
- De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012 Aug;97(8):2543-65. doi: 10.1210/jc.2011-2803. Erratum in: J Clin Endocrinol Metab. 2021 May 13;106(6):e2461. PMID: 22869843.
- van Raaij JM, Vermaat-Miedema SH, Schonk CM, Peek ME, Hautvast JG. Energy requirements of pregnancy in The Netherlands. Lancet. 1987 Oct 24;2(8565):953-5. doi: 10.1016/s0140-6736(87)91431-0. PMID: 2889869.
- Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997 Jun;18(3):404-33. doi: 10.1210/edrv.18.3.0300. PMID: 9183570.
- Organization WH. The global prevalence of anaemia in 2011. Geneva Switzerland WHO. 2011;126(11):5409–18.
- Tan J, He G, Qi Y, Yang H, Xiong Y, Liu C, Wang W, Zou K, Lee AH, Sun X, Liu X. Prevalence of anemia and iron deficiency anemia in Chinese pregnant women (IRON WOMEN): a national cross-sectional survey. BMC Pregnancy Childbirth. 2020 Nov 7;20(1):670. doi: 10.1186/s12884-020-03359-z. PMID: 33160312; PMCID: PMC7648278.
- World Bank. Prevalence of anaemia among pregnant women: Knoema; 2016. https://knoema.com/WBHNPS2018DEC/health-nutrition-and-population-statistics?tsId=1728550
- Wu Y, Ye H, Liu J, Ma Q, Yuan Y, Pang Q, Liu J, Kong C, Liu M. Prevalence of anemia and sociodemographic characteristics among pregnant and non-pregnant women in southwest China: a longitudinal observational study. BMC Pregnancy Childbirth. 2020 Sep 14;20(1):535. doi: 10.1186/s12884-020-03222-1. PMID: 32928139; PMCID: PMC7488658.
- Chakona G, Shackleton C. Food Taboos and Cultural Beliefs Influence Food Choice and Dietary Preferences among Pregnant Women in the Eastern Cape, South Africa. Nutrients. 2019 Nov 5;11(11):2668. doi: 10.3390/nu11112668. PMID: 31694181; PMCID: PMC6893604.
- Lammert F, Marschall HU, Glantz A, Matern S. Intrahepatic cholestasis of pregnancy: molecular pathogenesis, diagnosis and management. J Hepatol. 2000 Dec;33(6):1012-21. doi: 10.1016/s0168-8278(00)80139-7. PMID: 11131439.
- Beuers U, Pusl T. Intrahepatic cholestasis of pregnancy--a heterogeneous group of pregnancy-related disorders? Hepatology. 2006 Apr;43(4):647-9. doi: 10.1002/hep.21156. PMID: 16557565.
- Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009 May 7;15(17):2049-66. doi: 10.3748/wjg.15.2049. PMID: 19418576; PMCID: PMC2678574.
- Bacq Y. Intrahepatic cholestasis of pregnancy. Clin Liver Dis 1999; 3:1.
- Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2014 Jul;124(1):120-133. doi: 10.1097/AOG.0000000000000346. PMID: 24901263.
- Dixon PH, Williamson C. The molecular genetics of intrahepatic cholestasis of pregnancy. Obstet Med. 2008 Dec;1(2):65-71. doi: 10.1258/om.2008.080010. Epub 2008 Dec 1. PMID: 27582788; PMCID: PMC4989713.
- Geenes V, Chappell LC, Seed PT, Steer PJ, Knight M, Williamson C. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology. 2014 Apr;59(4):1482-91. doi: 10.1002/hep.26617. Epub 2014 Feb 26. PMID: 23857305; PMCID: PMC4296226.
- Kondrackiene J, Beuers U, Zalinkevicius R, Tauschel HD, Gintautas V, Kupcinskas L. Predictors of premature delivery in patients with intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2007 Dec 14;13(46):6226-30. doi: 10.3748/wjg.v13.i46.6226. PMID: 18069764; PMCID: PMC4171234.
- Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology. 2004 Aug;40(2):467-74. doi: 10.1002/hep.20336. PMID: 15368452.
- Locatelli A, Zagarella A, Toso L, Assi F, Ghidini A, Biffi A. Serial assessment of amniotic fluid index in uncomplicated term pregnancies: prognostic value of amniotic fluid reduction. J Matern Fetal Neonatal Med. 2004 Apr;15(4):233-6. doi: 10.1080/14767050410001668671. PMID: 15280130.
- Hou L, Wang X, Hellerstein S, Zou L, Ruan Y, Zhang W. Delivery mode and perinatal outcomes after diagnosis of oligohydramnios at term in China. J Matern Fetal Neonatal Med. 2020 Jul;33(14):2408-2414. doi: 10.1080/14767058.2018.1553944. Epub 2018 Dec 13. PMID: 30486718.
- Moore TR. Clinical assessment of amniotic fluid. Clin Obstet Gynecol. 1997 Jun;40(2):303-13. doi: 10.1097/00003081-199706000-00007. PMID: 9199842.
- Duff P. Premature rupture of the membranes in term patients. Semin Perinatol. 1996 Oct;20(5):401-8. doi: 10.1016/s0146-0005(96)80007-3. PMID: 8912994.
- Mercer BM, Arheart KL. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet. 1995 Nov 11;346(8985):1271-9. doi: 10.1016/s0140-6736(95)91868-x. Erratum in: Lancet 1996 Feb 10;347(8998):410. PMID: 7475723.
- Zhuang L, Li ZK, Zhu YF, Ju R, Hua SD, Yu CZ, Li X, Zhang YP, Li L, Yu Y, Zeng W, Cui J, Chen XY, Peng JY, Li T, Feng ZC. The correlation between prelabour rupture of the membranes and neonatal infectious diseases, and the evaluation of guideline implementation in China: a multi-centre prospective cohort study. Lancet Reg Health West Pac. 2020 Sep 17;3:100029. doi: 10.1016/j.lanwpc.2020.100029. PMID: 34327382; PMCID: PMC8315451.
- Karat C, Madhivanan P, Krupp K, Poornima S, Jayanthi NV, Suguna JS, Mathai E. The clinical and microbiological correlates of premature rupture of membranes. Indian J Med Microbiol. 2006 Oct;24(4):283-5. doi: 10.4103/0255-0857.29388. PMID: 17185848.
- Steer P. The epidemiology of preterm labour. BJOG. 2005 Mar;112 Suppl 1:1-3. doi: 10.1111/j.1471-0528.2005.00575.x. PMID: 15715585.
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009 Jun;33(3):130-7. doi: 10.1053/j.semperi.2009.02.010. PMID: 19464502.
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014 Jun;2(6):e323-33. doi: 10.1016/S2214-109X(14)70227-X. Epub 2014 May 5. PMID: 25103301.
- Chowa PE, Lin C, Goma F. Prevalence of Hypertension Among Women of Child 2011.
- Yang Y, Le Ray I, Zhu J, Zhang J, Hua J, Reilly M. Preeclampsia Prevalence, Risk Factors, and Pregnancy Outcomes in Sweden and China. JAMA Netw Open. 2021 May 3;4(5):e218401. doi: 10.1001/jamanetworkopen.2021.8401. PMID: 33970258; PMCID: PMC8111481.
- English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control. 2015 Mar 3;8:7-12. doi: 10.2147/IBPC.S50641. PMID: 25767405; PMCID: PMC4354613.
- Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005 Mar 12;330(7491):565. doi: 10.1136/bmj.38380.674340.E0. Epub 2005 Mar 2. PMID: 15743856; PMCID: PMC554027.
- Poon LC, Kametas NA, Chelemen T, Leal A, Nicolaides KH. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. J Hum Hypertens. 2010 Feb;24(2):104-10. doi: 10.1038/jhh.2009.45. Epub 2009 Jun 11. PMID: 19516271.
- Silva LM, Coolman M, Steegers EA, Jaddoe VW, Moll HA, Hofman A, Mackenbach JP, Raat H. Low socioeconomic status is a risk factor for preeclampsia: the Generation R Study. J Hypertens. 2008 Jun;26(6):1200-8. doi: 10.1097/HJH.0b013e3282fcc36e. PMID: 18475158.
- MATERNAL AND HEALTH SYSTEM PREDICTORS OF PREECLAMPSIA AMONG PREGNANT WOMEN ATTENDING HEALTH CARE FACILITIES IN LUSAKA, ZAMBIA: A RETROSPECTIVE COHORT STUDY. 2013. https://www.texilajournal.com/public-health/article/1860-maternal-and-health
- World Health Organization. WHO Recommendations for prevention and treatment of pre-eclampsia and eclampsia 2011.
- Visintin C, Mugglestone MA, Almerie MQ, Nherera LM, James D, Walkinshaw S; Guideline Development Group. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010 Aug 25;341:c2207. doi: 10.1136/bmj.c2207. PMID: 20739360.
- Roberts JM, Pearson G, Cutler J, Lindheimer M; NHLBI Working Group on Research on Hypertension During Pregnancy. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003 Mar;41(3):437-45. doi: 10.1161/01.HYP.0000054981.03589.E9. Epub 2003 Feb 10. PMID: 12623940.
- Koual M, Abbou H, Carbonnel M, Picone O, Ayoubi JM. Short-term outcome of patients with preeclampsia. Vasc Health Risk Manag. 2013;9:143-8. doi: 10.2147/VHRM.S38970. Epub 2013 Apr 15. PMID: 23610524; PMCID: PMC3629867.
- Savitz DA, Danilack VA, Engel SM, Elston B, Lipkind HS. Descriptive epidemiology of chronic hypertension, gestational hypertension, and preeclampsia in New York State, 1995-2004. Matern Child Health J 2013; 18: 829-838.
- Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998 Nov;179(5):1359-75. doi: 10.1016/s0002-9378(98)70160-7. PMID: 9822529.
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009 Jun;33(3):130-7. doi: 10.1053/j.semperi.2009.02.010. PMID: 19464502.
- WHO. Coverage of Maternity Care: A Listing of Available Information.Geneva, Switzerland: World Health Organization. 2004.
- Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, Jia C, Megson IL, Wei J, Zhang W. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS One. 2014 Jun 17;9(6):e100180. doi: 10.1371/journal.pone.0100180. PMID: 24937406; PMCID: PMC4061123.
- Lal AK, Gao W, Hibbard JU. Eclampsia: Maternal and neonatal outcomes. Pregnancy Hypertens. 2013 Jul;3(3):186-90. doi: 10.1016/j.preghy.2013.04.013. Epub 2013 Apr 24. PMID: 26106032.
- Mbu RE, Nana DPN. Maternal-fetal outcome in eclampic women delivered by caesarean or by vaginal route of the three tertiary hospitals in Yaounde. Clinics in Mother and Child Health. 2006;3(2):555562
- Priso EB, Njamen TN, Tchente CN, Kana AJ, Landry T, Tchawa UF, Hentchoya R, Beyiha G, Halle MP, Aminde L, Dzudie A. Trend in admissions, clinical features and outcome of preeclampsia and eclampsia as seen from the intensive care unit of the Douala General Hospital, Cameroon. Pan Afr Med J. 2015 Jun 9;21:103. doi: 10.11604/pamj.2015.21.103.7061. PMID: 26523163; PMCID: PMC4613832.
- Onuh SO, Aisien AO. Maternal and fetal outcome in eclamptic patients in Benin City, Nigeria. J Obstet Gynaecol. 2004 Oct;24(7):765-8. doi: 10.1080/01443610400009451. PMID: 15763783.
- Tikkanen M. Placental abruption: epidemiology, risk factors and consequences. Acta Obstet Gynecol Scand. 2011 Feb;90(2):140-9. doi: 10.1111/j.1600-0412.2010.01030.x. Epub 2010 Dec 7. PMID: 21241259.
- Mukherjee S, Bawa AK, Sharma S, Nandanwar YS, Gadam M. Retrospective study of risk factors and maternal and fetal outcome in patients with abruptio placentae. J Nat Sci Biol Med. 2014 Jul;5(2):425-8. doi: 10.4103/0976-9668.136217. PMID: 25097428; PMCID: PMC4121928.
- Bibi S, Ghaffar S, Pir MA, Yousfani S. Risk factors and clinical outcome of placental abruption: a retrospective analysis. J Pak Med Assoc. 2009 Oct;59(10):672-4. PMID: 19813679.
- Macheku GS, Philemon RN, Oneko O, Mlay PS, Masenga G, Obure J, Mahande MJ. Frequency, risk factors and feto-maternal outcomes of abruptio placentae in Northern Tanzania: a registry-based retrospective cohort study. BMC Pregnancy Childbirth. 2015 Oct 7;15:242. doi: 10.1186/s12884-015-0678-x. PMID: 26446879; PMCID: PMC4597387.
- Jabeen M, Gul F. Abruptio placentae: risk factors and perinatal outcome. J Postgrad3 Med Inst. 2011;18(4):669-76.
- Ananth CV, Cnattingius S. Influence of maternal smoking on placental abruption in successive pregnancies: a population-based prospective cohort study in Sweden. Am J Epidemiol. 2007 Aug 1;166(3):289-95. doi: 10.1093/aje/kwm073. Epub 2007 Jun 4. PMID: 17548787.
- Guo GL, Zhang YK, Li YL, Wang XX, Yang Y, Yu C, Wang L. [Epidemiological characteristics and related risk factors on placental abruption in Hebei province]. Zhonghua Liu Xing Bing Xue Za Zhi. 2018 Dec 10;39(12):1621-1625. Chinese. doi: 10.3760/cma.j.issn.0254-6450.2018;12:016. PMID: 30572389.
- Shen TT, DeFranco EA, Stamilio DM, Chang JJ, Muglia LJ. A population-based study of race-specific risk for placental abruption. BMC Pregnancy Childbirth. 2008 Sep 12;8:43. doi: 10.1186/1471-2393-8-43. PMID: 18789147; PMCID: PMC2546363.
- Wing DA, Fassett MJ, Getahun D. Acute pyelonephritis in pregnancy: an 18-year retrospective analysis. Am J Obstet Gynecol. 2014 Mar;210(3):219.e1-6. doi: 10.1016/j.ajog.2013.10.006. Epub 2013 Oct 5. PMID: 24100227.
- Yeta KI, Michelo C, Jacobs C. Antimicrobial Resistance among Pregnant Women with Urinary Tract Infections Attending Antenatal Clinic at Levy Mwanawasa University Teaching Hospital (LMUTH), Lusaka, Zambia. Int J Microbiol. 2021 Mar 4;2021:8884297. doi: 10.1155/2021/8884297. PMID: 33747088; PMCID: PMC7952174.