More Information
Submitted: April 14, 2023 | Approved: May 01, 2023 | Published: May 02, 2023
How to cite this article: Vu A, Moaddel V, Emmerich B, Rossman L, Bach J, et al. Association between the victim’s menstrual cycle phase and genital injuries following sexual assault. Clin J Obstet Gynecol. 2023; 6: 038-042.
DOI: 10.29328/journal.cjog.1001127
Copyright License: © 2023 Vu A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Sexual assault; Menstrual cycle; Follicular phase; Genital injuries; Epidemiology
Association between the victim’s menstrual cycle phase and genital injuries following sexual assault
Annie Vu1, Victoria Moaddel1, Bradley Emmerich2, Linda Rossman3, Jennifer Bach2, Jason Seamon2, Mariah Barnes2, Lindsey Ouellette1 and Jeffrey Jones1,2*
1Michigan State University College of Human Medicine, Department of Emergency Medicine, Grand Rapids, MI, USA
2Spectrum Health - Michigan State University Emergency Medicine Residency Program, Grand Rapids, MI, USA
3YWCA West Central Michigan Nurse Examiner Program, Grand Rapids, MI, USA
*Address for Correspondence: Jeffrey Jones, MD, 15 Michigan St NE Suite 736A, Grand Rapids, MI 49503, USA, (616) 234-2873, Email: [email protected]
Background: It is unknown what effect the menstrual cycle can have on the susceptibility to trauma following sexual assault.
Objectives: To compare the incidence of genital injuries following sexual assault in women with relationship to the three phases of the menstrual cycle
Methods: The design was a retrospective, cohort analysis set in a community-based nurse examiner program over a five-year study period. Sexual assault victims were between the ages of 13 - 40 years and agreed to a forensic examination. The menstrual cycle was divided into three phases: follicular, luteal and menses phase. The primary outcomes were the frequency and type of genital injuries documented in relation to the different phases of the menstrual cycle.
Results: Case files of 1376 cases of sexual assault were reviewed; 682 (49.6%) met the inclusion criteria. A total of 220 victims (32.3%) were in the follicular phase, 361 (52.9%) were in the luteal phase and 101 (14.8%) were in the menses phase. The three groups were comparable in terms of demographics, assault characteristics, and overall frequency of non-genital injuries. Assault victims in the follicular phase of the menstrual cycle had significantly more documented genital injuries (72.3%; 95% CI 66.4 - 78.2) compared to the luteal phase (64.0%; 95% CI 59.0 - 68.9) and the menses phase (58.4%; 95% CI 48.8 - 68.0).
Conclusion: Forensic examiners documented more genital injuries in the follicular phase of the menstrual cycle. Sex hormones may have confounding effects through influences on vaginal epithelial and mucosal integrity.
The forensic examination of sexual assault victims is performed to detect and treat trauma, as well as collect specimens and document injuries for prosecution. Colposcopy with digital imaging and staining with toluidine blue has led to reports of anogenital injury prevalence from 64% to 83% [1]. Prevalence rates may vary depending on examiner training and experience, examination type, age of the victim, the force of penetration, number of assailants and sedative or alcohol use within hours of the sexual assault [2,3]. While little is known about the role of female hormones in the potential for anogenital injury during sexual assault, estrogen and progesterone may have confounding effects through influences on vaginal epithelial and mucosal integrity [2]. For example, the menstrual cycle has long been considered a factor that could alter injury risk in female athletes [4]. Cyclical fluctuations in sex hormones such as estrogen and progesterone can influence musculoskeletal tissues such as ligaments, tendons and muscles. A recent study by Martin, et al. reported that muscle and tendon injuries in international footballers occurred almost twice as often in the days preceding ovulation, or the late follicular phase [5]. They even suggested that female athletes should monitor menstrual cycle length to determine when they are at increased risk for musculoskeletal injury. Several other studies have found a greater risk of Anterior Cruciate Ligament (ACL) injuries in the late follicular/ovulatory phase when estrogen concentrations are highest [6,7]. To date, the impact of menstrual cycle phases on genital injuries following sexual assault has not been explored.
The aim of this study was to compare the incidence of genital injuries following sexual assault in women with a relationship to the three phases of the menstrual cycle: follicular or preovulatory, luteal and menses.
Study design/setting
We conducted a retrospective, cohort analysis in conjunction with a community-based Nurse Examiner Program (NEP) over a five-year study period. Sexual assault victims presenting directly to any of the four downtown Emergency Departments (ED) are routinely referred to the NEP for evaluation after triage and initial assessment. The NEP is staffed by forensic nurses trained to perform medicolegal examinations using colposcopy with nuclear staining [8]. The nurse examiners at the NEP have completed a specialized Sexual Assault Nurse Examiner (SANE) program which includes a minimum of 40 hours of didactic and 40 hours of clinical training. Each nurse had performed over 200 sexual assault examinations prior to study initiation. An Institutional Review Board (IRB) affiliated with the NEP program approved this retrospective observational study.
Procedures
Nurse examiners identified anogenital injuries via visual inspection, magnification with colposcopy, and toluidine blue contrast application. The following anatomical sites are routinely evaluated and photographed for the presence and type of injury: external genitalia (thigh, mons pubis, labia majora, and perineum), internal genitalia (labia minora, periurethral area, posterior fourchette, fossa navicularis, hymen, cervix and vagina), and anus. Anoscopy is performed at the examiner’s discretion. Examiners used the TEARS (Tear, Ecchymosis, Abrasion, Redness, Swelling) classification to document injuries [1,9]. The number of genital and nongenital injuries was determined by simply counting each injury occurrence and totaling the count for each individual. Injury frequency was the number of genital or nongenital injuries sustained by each woman.
Population
Eligible patients were assault victims between the ages of 13 - 40 years who consented to a forensic examination and reported a regular menstrual cycle. Women were excluded if they were found to be pregnant, used hormonal birth control, had a history of irregular menstrual cycles, had missing or incomplete documentation, were examined more than 72 hours following the assault, or were unable to accurately recall their Last Menstrual Period (LMP). Women over 40 years were excluded to eliminate those in perimenopause, which can often precede menopause by years [10]. Women with vulvovaginal dermatoses (e.g. lichen planus) which might predispose them to vaginal injury were also excluded.
Data collection
Medical records were reviewed by three forensic nurses with training in research methodology. Research staff was taught how to perform data abstraction using a set of mock case records. One of the investigators met often with the abstractors to resolve questions and ensured that data variable definitions were uniformly applied. Patient demographics, menstrual cycle phase, assault characteristics and injury patterns were recorded using a standardized classification system.
Study definitions
Stringent criteria were used to define the menstrual cycle. Menstrual cycle phases were identified using self-reported days since their LMP. Patients were categorized into three groups: the follicular phase (the 9-day period following cessation of menses), the luteal phase (beginning 15 days after LMP) and the menses phase [2,11].
Statistical analyses
The primary outcome measure was the frequency and type of genital injuries documented with a relationship to the separate phases of the menstrual cycle. Data were entered into a Microsoft Excel database (Microsoft Corp, Redmond, WA, USA). All analyses were performed using SAS statistical software (SAS Institute, Cary, NC, USA). One investigator performed a critical review of a random sample of 10% of the charts to determine data reliability using the Kappa reliability test. Descriptive statistics were used to describe the frequency, location and type of anogenital injury according to the TEARS classification system. Chi-square and analysis of variance (ANOVA) tests were used to compare anogenital injuries in the examined victims; 95% Confidence Intervals (CI) were used to quantify uncertainty.
Case files of 1376 cases of sexual assault were reviewed; 682 (49.6%) met the inclusion criteria. A total of 220 victims (32.3%) were in the follicular phase, 361 (52.9%) were in the luteal phase, and 101 (14.8%) were in the menses phase of the menstrual cycle. The three groups were comparable in terms of demographics, parity, assault characteristics, and overall frequency of non-genital injuries (Table 1). Assault victims in the follicular phase of the menstrual cycle had significantly more documented genital injuries (72.3%; 95% CI 66.4 - 78.2) compared to the luteal phase (64.0%; 95% CI 59.0 - 68.9) and the menses phase (58.4%; 95% CI 48.8 - 68.0). These differences were statistically significant using the chi-square test for multiple categories (p = 0.022).
| Table 1: Patient demographics and assault characteristics. | ||||
| Menstrual Cycle | ||||
| Menstrual | Follicular | Luteal | p - Value | |
| Total # | 101 (14.8%) | 220 (32.3%) | 361 (52.9%) | |
| Age of victim, mean years | 22.1 ± 7.5 | 21.7 ± 7.9 | 23.0 ± 8.1 | 0.144 |
| Ethnicity (% white) | 75 (74.3%) | 167 (75.9%) | 271 (75.1%) | 0.946 |
| Marital status (% single) | 78 (77.2%) | 161 (73.2%) | 256 (70.9%) | 0.806 |
| Time interval to the exam, mean h | 21.8 ± 22.1 | 20.0 ± 20.9 | 21.0 ± 26.8 | 0.742 |
| Alcohol or drug use <24h | 55 (54.5%) | 130 (59.1%) | 203 (56.2%) | 0.848 |
| No prior history of sexual intercourse | 7 (6.9%) | 16 (7.3%) | 22 (6.1%) | 0.563 |
| Previous sexual assault | 45 (44.6%) | 87 (39.5%) | 140 (38.8%) | 0.573 |
| Police report filed | 78 (77.2%) | 164 (74.5%) | 285 (79.0%) | 0.470 |
| Known assailant | 85 (84.2%) | 191 (86.8%) | 322 (89.2%) | 0.353 |
| Multiple assailants | 12 (11.9%) | 29 (13.2%) | 39 (10.8%) | 0.688 |
| Type of sexual assault | ||||
| Vaginal | 76 (75.3%) | 174 (79.1%) | 289 (80.1%) | 0.577 |
| Oral | 27 (26.7%) | 63 (28.6%) | 101 (28.0%) | 0.940 |
| Anal | 14 (13.9%) | 28 (12.7%) | 45 (12.5%) | 0.933 |
| Digital | 28 (27.7%) | 81 (36.8%) | 129 (35.7%) | 0.252 |
| Non-genital injuries | 41 (40.6%) | 93 (42.3%) | 143 (39.6%) | 0.818 |
| Anogenital injuries | 58 (58.4%) | 157 (72.3%) | 231 (64.0%) | 0.021 |
| Anogenital injuries, total | 162 | 462 | 614 | |
| Anogenital injuries, mean (SD) | 1.6 ± 1.9 | 2.1 ± 2.0 | 1.7 ± 1.8 | 0.022 |
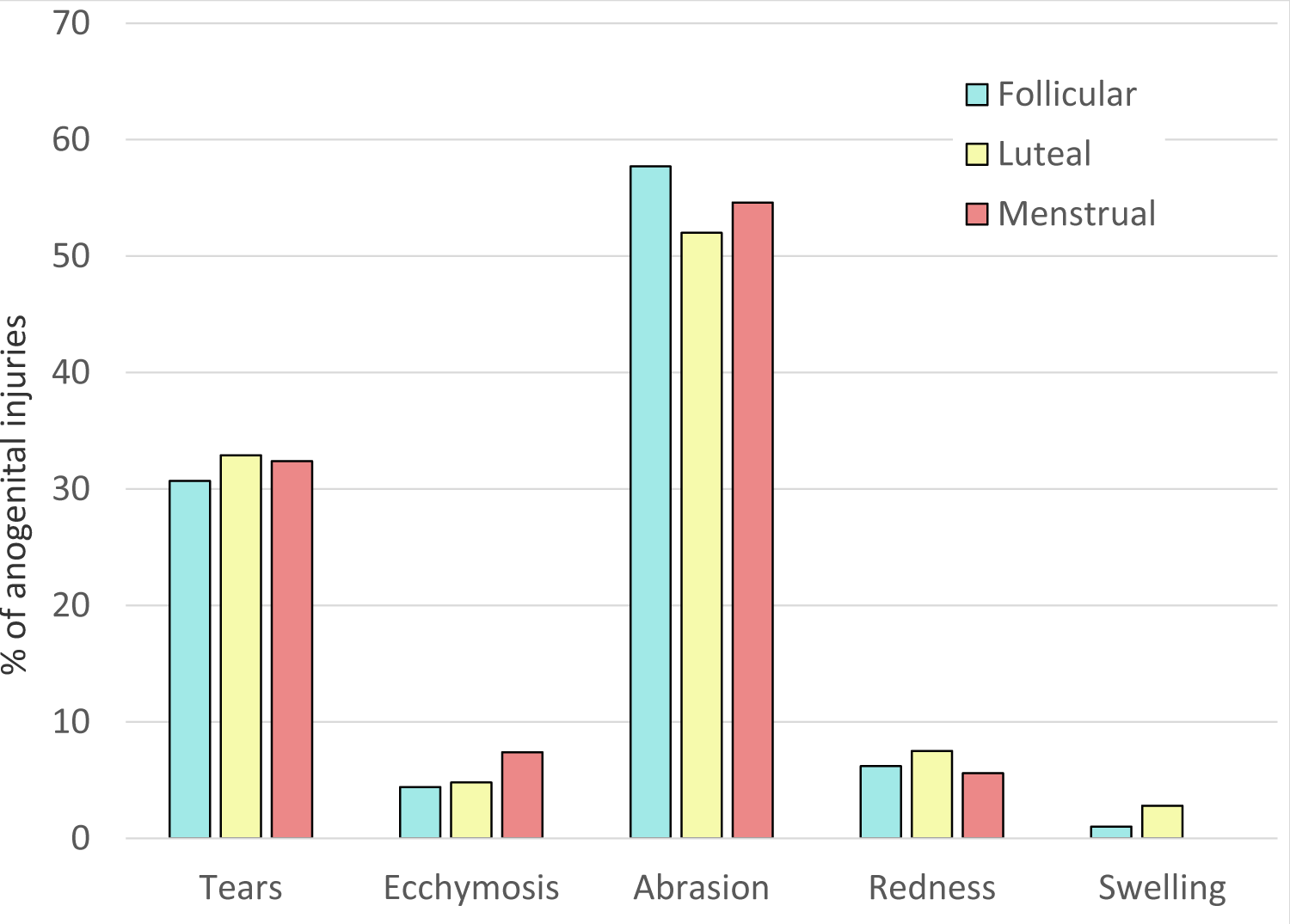
Using the TEARS classification system, the localized pattern, type, and frequency of anogenital injuries did not vary significantly between the three phases of the menstrual cycle (Figure 1). The common sites of injury were located posteriorly, including the fossa navicularis, posterior fourchette, labia minora and anus. The interrater agreement for this sample of charts was excellent (K statistic = 0.91).
Figure 1: Types of genital trauma in relation to menstrual cycle.
This is the first clinical study to compare the documentation of genital injuries following sexual assault in women with a relationship to the separate phases of the menstrual cycle. Assault victims in the follicular phase of the menstrual cycle had significantly more documented anogenital injuries compared to the luteal phase or the menses phase. The follicular phase includes the stimulation and development of ovarian follicles via Follicle-Stimulating Hormone (FSH) and Luteinizing Hormone (LH) [12]. Estrogen levels are high during this phase as estrogen increases the growth of the endometrium and stimulates the emergence of spiral arteries. At the end of the follicular phase and nearing ovulation, estrogen levels are the highest due to follicle maturation. This leads to an increase in FSH and LH levels, called the LH surge, which causes an oocyte to be released. The next phase is the luteal phase and progesterone, stimulated by LH, is the dominant hormone involved in this phase as it prepares the corpus luteum and endometrium for possible fertilized ovum implantation. If no fertilized ovum is implanted, the levels of estrogen, progesterone, FSH and LH decrease and lead to menstruation with atresia of the corpus lutem and endometrium [12].
Little is known about the role of sex hormones, estrogen, and progesterone, in the potential for anogenital injury during coitus. However, estrogen regulates host immune responses, increases glycosaminoglycans and collagen production and affects skin thickness as well as tissue integrity [2,13]. Older or postmenopausal women have more anogenital injuries due to their decreased estrogen levels and loss of tissue elasticity [14]. A study of women following consensual sexual intercourse found that among menstruating women, those in the follicular phase had a greater prevalence and frequency of genital injuries than those in other phases [2]. The same study also reported that women using hormonal birth control had 38% more genital injuries and more than twice the anal injuries as the non-hormonal birth control menstruating group. These findings are similar to a study by Sommers, et al. who found that the odds of anogenital injury after consensual sexual intercourse among women who used hormonal birth control were 2.5 times greater than among women who used no birth control [15]. Other studies suggest that female sex hormones facilitate the transmission of sexually transmitted infections (STIs) by altering vaginal epithelial and mucosal barrier integrity [2,16,17]. Both the phase of the menstrual cycle and the use of hormonal contraceptives have been shown to influence susceptibility to initial infection with chlamydia, gonorrhea, herpes simplex, and human immunodeficiency virus in women [16,17].
We also found that menstruating women had 6% - 14% fewer documented anogenital injuries when compared to non-menstruating victims (Table 1). This is consistent with an earlier retrospective study from our institution which examined 177 cases of sexual assault in menstruating women matched (1:4) with non-menstruating sexual assault victims presenting to the same clinic [13]. Their report demonstrated that menstruating victims had 11% fewer anogenital lacerations and 12% fewer abrasions and ecchymoses than non-menstruating women. They concluded that the presence of menstrual blood during colposcopic examination likely masked anogenital injuries such as superficial lacerations and abrasions. An alternative explanation is that sex hormone levels could have confounding effects through influences on vaginal epithelial and mucosal integrity [2].
Research has consistently shown that documented physical injury influences decision-making and legal outcomes throughout the criminal justice process. Because the examination is based on scientific evidence, it may influence victims to report their experiences to the police, encourage police to file a complaint, and persuade prosecutors to file sexual assault charges and pursue a conviction [18,19]. For example, McGregor, et al. demonstrated that the presence of genital trauma was significantly related to the filing of charges by the prosecutor as well as conviction [20]. Predisposing or etiologic factors specific to genital injury following sexual assault in women are not well defined in the literature. The factors listed in Table 2 are characteristics associated with genital trauma in general. This broad assortment is likely affected by examiner training and experience, differences in injury definitions, patient population, as well as exam technique. The novel findings from this study may contribute to this list and may account for the greater number of genital injuries in documented in postmenopausal women [14].
| Table 2: Known predisposing factors for genital injury following sexual assault [3,9,13,14,18]. |
| The assailant was known to the victim |
| Presence of nongenital injuries |
| Alcohol or drug use by an assailant |
| Physical coercion |
| Digital penetration |
| Use of Weapons |
| Adolescent postmenopausal victims |
| Multiple assailants |
| Location of assault outdoors |
| No prior sexual intercourse |
| Preexisting vaginal infection |
| Insertion of foreign bodies |
| Previous anogenital surgery |
This small retrospective study can show association but cannot be used to prove causation. Prospective studies will be needed to establish the causative link between the menstrual cycle phase and genital injuries after sexual assault. The study design also prevented the control for clinical evaluations by different forensic examiners. It could be that documentation was not uniform, although all the nurse examiners had a similar level of training and experience. Injury prevalence was calculated based on injury identification by three methods: visual inspection, colposcopic magnification and toluidine blue staining. Although these methods are considered state-of-the-art, human error may have led to injury misidentification. The findings of the examiners were recorded on state-mandated reporting forms and were taken as the most accurate representation of the actual physical findings. Since the LMP was self-reported by the victims, the identification of the different phases of the menstrual cycle could not be verified and may be inaccurate. This study, like all studies of sexual assault victims, is vulnerable to sample bias. Feldhaus, et al. [21] reported that fewer than half of sexual assault victims reported the assault to the police or sought medical care. Because the data in this study are based on victims presenting for medical evaluation, they may not be completely generalizable to all cases of sexual assault. Therefore, it is possible that the proportion of sexual assaults involving significant force and injuries may be overrepresentation. Finally, the presence of menstrual bleeding might also affect the identification of anogenital injury in the menses phase [13]. Despite these limitations, it seems reasonable to theorize that the different phases of the menstrual cycle influence the frequency of documented anogenital injuries following sexual assault.
This is the first clinical study to compare the documentation of genital injuries following sexual assault in women with a relationship to the different phases of the menstrual cycle. Using colposcopy with nuclear staining, forensic examiners consistently documented more anogenital injuries in the follicular phase of the menstrual cycle which is characterized by increasing amounts of estrogen. While little is known about the role of female hormones in the potential for anogenital injury during the sexual assault, sex hormones may have confounding effects through influences on vaginal epithelial and mucosal integrity. Further research is needed to investigate biological factors that influence the incidence of anogenital injuries following sexual assault in women.
- Sommers MS. Defining patterns of genital injury from sexual assault: a review. Trauma Violence Abuse. 2007 Jul;8(3):270-80. doi: 10.1177/1524838007303194. PMID: 17596344; PMCID: PMC3142744.
- Brawner BM, Sommers MS, Moore K, Aka-James R, Zink T, Brown KM, Fargo JD. Exploring Genitoanal Injury and HIV Risk Among Women: Menstrual Phase, Hormonal Birth Control, and Injury Frequency and Prevalence. J Acquir Immune Defic Syndr. 2016 Feb 1;71(2):207-12. doi: 10.1097/QAI.0000000000000824. PMID: 26334741; PMCID: PMC4712081.
- Rossman L, Solis S, Ouellette L, Kolacki C, Jones JS. Vulvovaginal Lacerations Following Consensual Versus Non-consensual Vaginal Penetration. Academic Emergency Medicine. 2021; 28 (S1):S391.
- Legerlotz K, Nobis T. Insights in the Effect of Fluctuating Female Hormones on Injury Risk-Challenge and Chance. Front Physiol. 2022 Feb 17;13:827726. doi: 10.3389/fphys.2022.827726. PMID: 35250631; PMCID: PMC8891628.
- Martin D, Timmins K, Cowie C, Alty J, Mehta R, Tang A, Varley I. Injury Incidence Across the Menstrual Cycle in International Footballers. Front Sports Act Living. 2021 Mar 1;3:616999. doi: 10.3389/fspor.2021.616999. Erratum in: Front Sports Act Living. 2021 Aug 18;3:745792. PMID: 33733235; PMCID: PMC7956981.
- Chidi-Ogbolu N, Baar K. Effect of Estrogen on Musculoskeletal Performance and Injury Risk. Front Physiol. 2019 Jan 15;9:1834. doi: 10.3389/fphys.2018.01834. PMID: 30697162; PMCID: PMC6341375.
- Wild CY, Steele JR, Munro BJ. Why do girls sustain more anterior cruciate ligament injuries than boys?: a review of the changes in estrogen and musculoskeletal structure and function during puberty. Sports Med. 2012 Sep 1;42(9):733-49. doi: 10.1007/BF03262292. PMID: 22784194.
- Rossman L, Dunnuck C. A community sexual assault program based in an urban YWCA: the Grand Rapids experience. J Emerg Nurs. 1999 Oct;25(5):424-7. doi: 10.1016/s0099-1767(99)70104-2. PMID: 10508471.
- Slaughter L, Brown CR, Crowley S, Peck R. Patterns of genital injury in female sexual assault victims. Am J Obstet Gynecol. 1997 Mar;176(3):609-16. doi: 10.1016/s0002-9378(97)70556-8. PMID: 9077615.
- Delamater L, Santoro N. Management of the Perimenopause. Clin Obstet Gynecol. 2018 Sep;61(3):419-432. doi: 10.1097/GRF.0000000000000389. PMID: 29952797; PMCID: PMC6082400.
- Draper CF, Duisters K, Weger B, Chakrabarti A, Harms AC, Brennan L, Hankemeier T, Goulet L, Konz T, Martin FP, Moco S, van der Greef J. Menstrual cycle rhythmicity: metabolic patterns in healthy women. Sci Rep. 2018 Oct 1;8(1):14568. doi: 10.1038/s41598-018-32647-0. Erratum in: Sci Rep. 2019 Apr 3;9(1):5797. PMID: 30275458; PMCID: PMC6167362.
- Thiyagarajan DK, Basit H, Jeanmonod R. Physiology, Menstrual Cycle. 2022 Oct 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 29763196.
- Rossman L, Solis S, Stevens J, Wynn B, Jones JS. Effect of menstrual bleeding on the detection of anogenital injuries in sexual assault victims. Am J Emerg Med. 2019 Jun;37(6):1203-1204. doi: 10.1016/j.ajem.2018.11.005. Epub 2018 Nov 6. PMID: 30415984.
- Jones JS, Rossman L, Diegel R, Van Order P, Wynn BN. Sexual assault in postmenopausal women: epidemiology and patterns of genital injury. Am J Emerg Med. 2009 Oct;27(8):922-9. doi: 10.1016/j.ajem.2008.07.010. PMID: 19857408.
- Sommers MS, Zink TM, Fargo JD, Baker RB, Buschur C, Shambley-Ebron DZ, Fisher BS. Forensic sexual assault examination and genital injury: is skin color a source of health disparity? Am J Emerg Med. 2008 Oct;26(8):857-66. doi: 10.1016/j.ajem.2007.11.025. PMID: 18926341; PMCID: PMC2587067.
- Kaushic C, Roth KL, Anipindi V, Xiu F. Increased prevalence of sexually transmitted viral infections in women: the role of female sex hormones in regulating susceptibility and immune responses. J Reprod Immunol. 2011 Mar;88(2):204-9. doi: 10.1016/j.jri.2010.12.004. Epub 2011 Feb 5. PMID: 21296427.
- Brabin L. Interactions of the female hormonal environment, susceptibility to viral infections, and disease progression. AIDS Patient Care STDS. 2002 May;16(5):211-21. doi: 10.1089/10872910252972267. PMID: 12055029.
- Sommers MS, Fisher BS, Karjane HM. Using colposcopy in the rape exam: health care, forensic, and criminal justice issues. J Forensic Nurs. 2005 Spring;1(1):28-34, 19. doi: 10.1111/j.1939-3938.2005.tb00008.x. PMID: 17073052.
- Gray-Eurom K, Seaberg DC, Wears RL. The prosecution of sexual assault cases: correlation with forensic evidence. Ann Emerg Med. 2002 Jan;39(1):39-46. doi: 10.1067/mem.2002.118013. PMID: 11782729.
- McGregor MJ, Du Mont J, Myhr TL. Sexual assault forensic medical examination: is evidence related to successful prosecution? Ann Emerg Med. 2002 Jun;39(6):639-47. doi: 10.1067/mem.2002.123694. PMID: 12023707.
- Feldhaus KM, Houry D, Kaminsky R. Lifetime sexual assault prevalence rates and reporting practices in an emergency department population. Ann Emerg Med. 2000 Jul;36(1):23-7. doi: 10.1067/mem.2000.107660. PMID: 10874231.