More Information
Submitted: August 01, 2022 | Approved: August 16, 2022 | Published: August 17, 2022
How to cite this article: Underkofler KA, Morell AJ, Esquivel R, De Simone FI, Miller MC, et al. Cost-analysis comparison of clinical risk assessment with and without ROMA for the management of women with pelvic masses. Clin J Obstet Gynecol. 2022; 5: 080-089.
DOI: 10.29328/journal.cjog.1001112
Copyright License: © 2022 Underkofler KA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: ROMA; Initial clinical risk assessment; Healthcare costs; Pelvic masses; HE4; CA125; Imaging
Cost-analysis comparison of clinical risk assessment with and without ROMA for the management of women with pelvic masses
Kaylee A Underkofler1, Alexandra J Morell1, Rianne Esquivel2, Francesca I DeSimone2, M Craig Miller3 and Richard G Moore1*
1Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, University of Rochester, Wilmot Cancer Institute, NY, USA
2Fujirebio Diagnostics Inc. Malvern, PA, USA
3Statistical Consultant, Fujirebio Diagnostics Inc. Malvern, PA, USA
*Address for Correspondence: Richard G Moore, Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, University of Rochester, Wilmot Cancer Institute, Rochester, NY 14620, USA, Email: [email protected]
Objective: Pelvic masses can be classified as low risk (likely benign) and high risk (likely malignant) based on an initial clinical risk assessment, which involves a detailed history, physical exam, basic laboratory tests, and imaging. In recent years, the Risk of Ovarian Malignancy Algorithm (ROMA), which combines CA125, HE4 and menopausal status, has emerged as a powerful tool in the classification of pelvic masses and triage of patients to either a generalist gynecologist or a gynecologic oncologist for management. The objective of this study was to evaluate whether the use of ROMA, alone or in combination with Initial Clinical Risk Assessment (ICRA), provides cost savings compared to triage based on ICRA alone.
Methods: A health-economic decision model was developed to assess clinical and cost differences associated with three different clinical pathways of risk assessment for a pelvic mass: ICRA alone, ROMA alone, or ICRA + ROMA in combination. Using previously reported accuracy rates and patient characteristics from a prospective, multicenter, blinded clinical trial, total healthcare costs were modeled for each clinical pathway using the Medicare 2020 reimbursement rates.
Results: A total of 461 patients with pelvic masses were included with 10.4% ultimately diagnosed with epithelial ovarian cancer. Total healthcare costs for patients with benign disease, EOC, or low malignant potential tumors (LMP) (n = 441) triaged using ROMA alone were 3.3% lower than when triaged using ICRA alone. While lab costs increased 55% using ROMA, the use of ROMA alone resulted in a 4% decrease in laparoscopy costs and a 3.1% decrease in laparotomy costs compared with ICRA alone. Similarly, total costs associated with a combination of ICRA + ROMA were 3.9% lower than total costs associated with ICRA alone. The model also predicted a 63% reduction in repeat surgeries resulting from false negative ICRA when using ROMA to triage patients.
Conclusion: Triage of women with pelvic masses using the more sensitive ROMA score lowers overall healthcare costs compared to ICRA alone. With fewer false negative results than ICRA alone, the ROMA score improves initial detection of malignancy and reduces second surgical treatments in women with pelvic masses.
Improvements in screening, diagnosis, and care for cancer patients are expected to increase the number of cancer survivors (from 13.8 to 18.1 million) between 2010 and 2020. The increase in survival is expected to be accompanied by a 27% increase in the cost of cancer care in the United States and cancer care costs were predicted to increase from $124.6 billion in 2010 to $157.8 billion by 2020 (in 2010 dollars) [1]. An additional study projected similar cancer-attributed medical care costs to reach $246 billion by 2030 in the United States [2]. The increase in cancer health care costs can be partially explained by increased cancer treatment intensity and maintenance therapy, duration of treatment, increased survival time, increased drug costs, advanced imaging [2] and rising costs of cancer-related surgeries and postoperative care [3]. Gynecological cancers place a considerable economic burden on society [4]. It has been estimated that in 2020 ovarian cancer had the highest costs ($6.03 billion) followed by uterine ($3.05 billion) and cervical ($1.54 billion) [1].
Ovarian cancer is a complex disease often treated with a combination of systemic treatment (or chemotherapy), surgery, and occasionally radiation [5]. Ovarian cancer is also characterized by a high rate of complications which can significantly impact the cost of care [5].
In the US, in 2010, the initial cost to treat ovarian cancer was estimated at $82,324 during the first year, while in the final year of life, the average cost per patient was estimated to be $99,715 [6]. An understanding of cancer care costs is an important step to evaluating the economic burden of unnecessary procedures, including repeated surgery, and to help identify the contributing factors driving cost increases. A deeper understanding of cancer-related costs can help minimize, if not eliminate, unwanted variations in care cost [3]. Extensive efforts have been made to create accurate and reliable tools to assess women with an ovarian cyst or pelvic mass for ovarian cancer. Accurate risk stratification is critical for women that are at high risk for harboring a malignancy as proper triage to a gynecologic oncologist and centers experienced in the management and care of women with ovarian cancer result in decreased morbidity and increased survival. The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) have specific recommendations for referral of women with a pelvic mass to a gynecologic oncologist (ACOG Practice Bulleting #174, 2016) [7]. Current modalities recommended for the evaluation of women with a pelvic mass include history and physical exam, imaging, biomarker analysis, and formal risk stratification algorithms such as the Risk of Ovarian Malignancy Algorithm (ROMA). Patients that are found to have a nodular or fixed pelvis mass, ascites, elevated CA125, ultrasound findings suggestive of malignancy, evidence of abdominal or distant metastasis (by examination or imaging study), and or an elevated score on a formal risk assessment test, such as ROMA, should be considered at high risk for ovarian cancer and referred to a gynecologic oncologist. In 2009, Moore, et al. reported on the Risk of Ovarian Malignancy Algorithm (ROMA) [8], an algorithm that combines serum measurements of CA 125, Human Epididymis Protein 4 (HE4), and the patient’s menopausal status to predict the risk of malignancy in women with a pelvic mass. Since the publication of the ROMA and the FDA clearance for its use for risk assessment of women with a pelvic mass, ROMA has become a standard test and valuable tool for risk assessment. ROMA is used to identify women with a high risk of having ovarian cancer and differentiate them from the low-risk group with mostly benign diseases [9]. This risk stratification helps ensure optimal patient care by promoting the triage of patients at high risk of ovarian malignancy to tertiary care centers with multidisciplinary teams that specialize in ovarian cancer and allow women at low risk to stay with their gynecologists for the appropriate management and care [9]. The performance characteristics of ROMA at a set specificity of 75%, include a sensitivity of 94% with a Negative Predictive Value (NPV) of 99.0% for the diagnosis of epithelial ovarian cancer (EOC) [8,10].
The current study aimed to evaluate the cost-effectiveness of ROMA, alone or in combination with Initial Clinical Risk Assessment (ICRA) compared with ICRA alone for the triage of women with adnexal masses being scheduled for surgery.
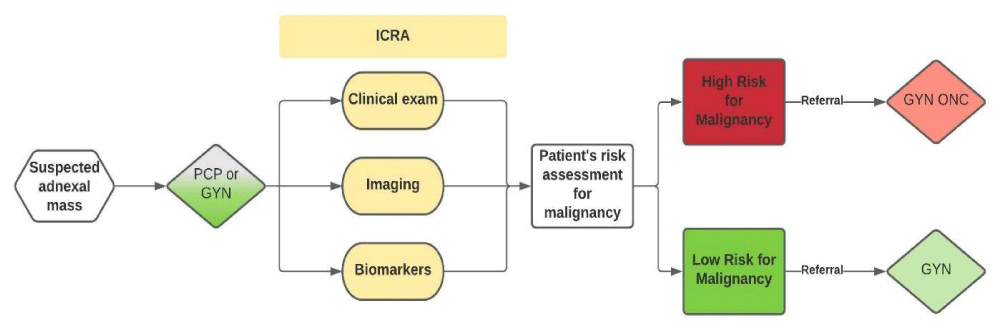
A health-economic decision model was developed to assess clinical and cost differences associated with procedures performed, visits to the provider, and referrals for patients presenting with an adnexal mass. This model was independently constructed by L3 Healthcare (San Diego, CA) and includes inputs from clinical trial data, published references, and clinical expert input. The model was developed by initially defining the patient care pathway (Figure 1), which begins with a presentation of a patient with a suspected adnexal mass to a provider, including primary care physicians (PCP) or gynecologists (GYN). According to the ACOG practice guidelines, individual patient characteristics, physical examination findings, imaging results (including ultrasound and CT- scans), and serum CA 125 measurements should all be used in combination for the evaluation and management of adnexal masses [7]. In the original FDA trial for ROMA clearance, all physicians were required to provide an Initial Clinical Risk Assessment (ICRA) assigning all patients with an adnexal mass as high versus low risk for ovarian cancer. The physicians were to use the ACOG criteria, which was the standard of care at the time of the trial and were blinded to HE4 and ROMA results. The ICRA in combination with a risk assessment algorithm (like ROMA) is quickly becoming the current standard of care in the US [11]. The ICRA plus a risk assessment algorithm allows physicians to determine whether surgery is necessary and more specifically, to determine if the patient is at low risk or high risk for malignancy. Patients considered to have a low risk for malignancy can ideally be managed by a gynecologist for surgery and for subsequent follow-up treatment, whereas patients considered to have a high risk for malignancy should be referred to a gynecologic oncologist.
Figure 1: Patient care pathway for women presenting with an adnexal mass.
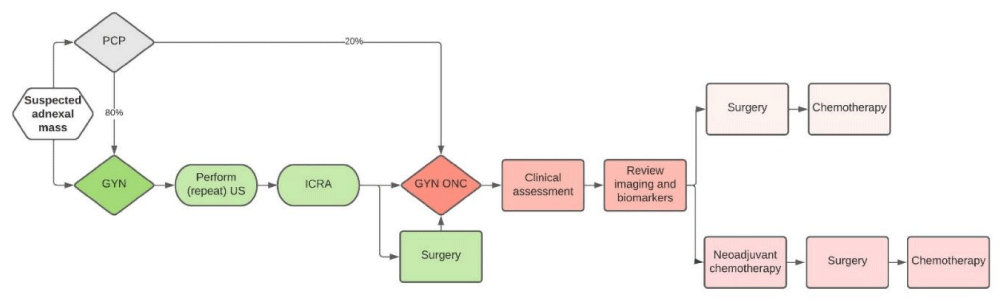
As shown in Figure 2, our health-economic decision model assumes that all patients presenting to a PCP with a suspected adnexal mass are subsequently referred out for surgery to either a generalist gynecologist (GYN) or to a gynecologic oncologist (GYN ONC). Based on expert opinion, when patients are initially evaluated by the PCP, only a small percentage (20%) are referred directly to a gynecologic oncologist, while the majority (80%) are referred to a GYN for further assessment and (in some cases) surgery. Upon referral to a gynecologist by the PCP, it is assumed that the gynecologist will often repeat pelvic ultrasound imaging and perform their own ICRA to classify patients and assess their risk of malignancy. However, it is assumed that the gynecologists will not repeat any lab testing (i.e. basic metabolic panel and complete blood counts) and/or advanced imaging such as CT scans or MRI scans if they have already been requested or performed by the initial ordering physician (i.e. the PCP). If the gynecologist ICRA based on the information they have available classifies a patient as being suspicious of malignancy or as having a high risk for malignancy, it is assumed that the gynecologist will then refer the patient to a gynecologic oncologist. Complications due to the treatments administered are not factored into the model as the difficulty of the case may be due to the case mix (i.e. the complicated nature of the case) vs. the surgeon’s risk of complications. However, general consensus suggests complication rates are higher when surgery is performed by non-gynecologic oncologists in institutions with lower procedure volumes [12,13]. Furthermore, our model does not include cases in which imaging was not performed by the initial ordering physician (either the PCP or GYN) and was subsequently performed by the referred physician.
Figure 2: Referral pathway for women presenting with an adnexal mass.
In clinical practice, when there is a discrepancy between the ICRA and ROMA results (i.e. ICRA indicated a low risk for malignancy while ROMA indicated a high risk for malignancy), the treating physician may repeat or order new imaging studies (i.e. CT and/or MRI scans). The impact of this clinical scenario is also not factored into the model. Finally, postsurgical treatments (including chemotherapy) are not included in the analysis as the impact does not affect the use of biomarkers at the time of the initial assessment for malignancy risk.
In this study we created a model using the assumptions described above to quantify the economic differences of three clinical pathways for risk assessment: 1) using ICRA alone; 2) using ROMA alone, and 3) using a combination of ICRA + ROMA as a determining factor for referral to a GYN ONC with either the ICRA and or ROMA indicating a high risk for malignancy resulting in a referral to a gynecologic oncologist. All pathways were consistent with the previously mentioned ACOG Practice guidelines for the evaluation and management of adnexal masses [10]. We used previously reported accuracy rates for ICRA, ROMA, and ICRA + ROMA, as well as the characteristics of the patient population, from a prospective, multicenter, blinded clinical trial (FDI-15, ClinicalTrial.gov identifier NCT00987649) to assess the triage of women with a pelvic mass using ICRA and/or ROMA and created a model reflecting the related lab testing, imaging, surgical procedures, and associated costs. All costs were estimated using 2020 Medicare reimbursement rates as shown in Table 1. The surgery costs used were the mean of all codes, with an increase in these costs associated with laparotomy and reoperation. Variation in resource utilization associated with the surgical management of ovarian cancer was based on published work by Rauh-Hain and colleagues. In their study, the authors indicated that laparotomy is associated with a 29% increase in cost, whereas reoperation is associated with a 49% increase in cost [3]. As previously indicated, the cost of complications was not added to this model but is a potential additional cost factor. The impact of costs on the patient is also not factored into the model.
| Table 1: Medicare codes. | ||
| Category | CMS Coding | Specification |
| CLFS 2020 | CPT 86304 | Tumor Antigen by Immunoassay CA 125 |
| CLFS 2020 | CPT 86305 | HE4, Ovarian Cancer Monitoring |
| CLFS 2020 | CPT 80053 | Comprehensive metabolic panel |
| CLFS 2020 | CPT 85027 | Blood counts |
| Office Visits - PCP/GP/GYN | CPT 99203 | Office/Outpatient visit, new, comprehensive H/E, low complexity DM (PCP/GP/GYN) |
| Office Visits - PCP/GP/GYN | CPT 99213 | Office/Outpatient visit, est. (PCP/GP/GYN) |
| Office Visits - GYN ONC | CPT 99205 | Office/Outpatient visit, new, comprehensive H/E, high complexity DM (GYN ONC) |
| Office Visits - GYN ONC | CPT 99214 | Office/outpatient visit est., (GYN ONC) |
| Surgeries | CPT 58661 | Laparoscopy removes adnexal mass (Minimally invasive BSO) |
| CT-scans | 74160 | Computed tomography, abdomen |
| CT-scans | 72193 | CT pelvis with contrast material(s) |
| CT-scans | 71260 | CT thorax with contrast material(s) |
| Ultrasound (US) Imaging | 76830 | No obstetric Pelvic Ultrasound |
| CLFS: Clinical Lab Fee Schedule; CMS: Centers for Medicare & Medicaid Services; CPT: Current Procedural Terminology | ||
In this model, total healthcare cost was determined by the combination of costs derived from total lab testing, total imaging (including US and CT scans requested by the clinician performing the initial assessment), and total surgery costs (including laparoscopy, laparotomy, and repeated surgery due to false negative results). Proportions of subjects falling into various groups of interest were determined and Fisher’s exact testing was used to calculate p-values for the comparison of the various proportions of interest. The predicted number of surgeries performed by a specialist following a referral that was based on ICRA, ROMA, and ICRA + ROMA, were determined based on expert opinion.
Effects on total healthcare costs
As previously mentioned, the main goal of this study was to evaluate the cost-effectiveness of using three different methods for the initial assessment of women with a pelvic mass to determine their risk of having ovarian cancer: ICRA, ROMA, or their combination (ICRA + ROMA). In this study, Epithelial Ovarian Cancer (EOC) includes primary ovarian, fallopian tube, and primary peritoneal cancers. The clinical study (FDI-15, ClinicalTrial.gov identifier NCT00987649) included a total of 461 patients, 375 (81.3%) with a benign pelvic mass, 18 (3.9%) with a borderline/low malignant potential (LMP) tumor, 48 (10.4%) with epithelial ovarian cancer (EOC) and 20 (4.3%) with other cancers (including non-epithelial ovarian cancers, other gynecologic cancers metastatic to the ovary and non-gynecologic cancers metastatic to the ovary) (Table 2).
| Table2: Characteristics of FDI-15 PatientPopulation (n = 461 patients). | ||||
| N | All461 | Pre-Menopausal240 (52.1%) | Post-Menopausal221 (47.9%) | Pre-Menopausal vs. Post-Menopausal |
| Age | T-Test p value | |||
| Mean± Std. Dev. [Median] | 50+ /- 15 [49] | 40+ /- 9 [42] | 62+ /- 10 [62] | 0.0000 |
| Race | Fisher’sExact p value | |||
| White | 391(84.8%) | 193(80.4%) | 198(89.6%) | 0.021 |
| Black | 31(6.7%) | 20(8.3%) | 11(5.0%) | |
| Other/Unknown | 39(8.5%) | 27(11.3%) | 12(5.4%) | |
| ICRA | Fisher’sExact p value | |||
| Benign | 339(73.5%) | 211(87.9%) | 128(57.9%) | 0.000 |
| Cancer | 122(26.5%) | 29(12.1%) | 93(42.1%) | |
| PathologyDiagnosis | Fisher’sExact p value | |||
| Benign | 375(81.3%) | 220(91.7%) | 155(70.1%) | 0.000 |
| Borderline/LMPTumor | 18(3.9%) | 7(2.9%) | 11(5.0%) | |
| Cancer– Epithelial Ovarian I-II | 12(2.6%) | 3(1.3%) | 9(4.1%) | |
| Cancer– Epithelial Ovarian III-IV | 34(7.4%) | 5(2.1%) | 29(13.1%) | |
| Cancer– Epithelial Ovarian Unstaged | 2(0.4%) | 1(0.4%) | 1(0.5%) | |
| Cancer– Non-Epithelial Ovarian | 2(0.4%) | 0(0.0%) | 2(0.9%) | |
| Cancer– Other Gynecological | 10(2.2%) | 3(1.3%) | 7(3.2%) | |
| Cancer– Other | 7(1.5%) | 1(0.4%) | 6(2.7%) | |
| Cancer– Metastatic | 1(0.2%) | 0(0.0%) | 1(0.5%) | |
We first assessed the impact of the ROMA score on total healthcare cost for patients triaged for a pelvic mass as opposed to the cost of ICRA alone. The use of ROMA for the triage of women with an adnexal mass in the group of subjects diagnosed with benign disease vs EOC + LMP (n = 441) resulted in a 3.3% reduction in total costs compared to ICRA’s costs ($2,559,012 vs. $2,646,915, respectively) Table 3, thus indicating a cost difference of $87,904 between the two assessment tools. Specifically, we observed a substantial decrease in surgery costs when the ROMA score was used for EOC + LMP risk assessment. We found that ROMA score led to a 4% reduction ($82,519) in total laparoscopy costs compared to ICRA alone ($2,000,065 vs. $2,082,584) as well as a 3.1% decrease ($14,562) in total laparotomy costs (ROMA score: $452,636; ICRA: $467,198). Taken together, the decrease in laparoscopy and laparotomy provides evidence that the use of the ROMA score as an alternative to ICRA for the triage of women suspected of ovarian cancer to a GYN ONC results in a 3.8% ($ 97,081) reduction in total surgery costs ($2,452,701 vs. $2,549,782). Similarly, in this same group of subjects, the combination of ICRA + ROMA also showed a decrease in costs compared to ICRA alone. Specifically, the combination of ROMA with ICRA showed a 4.6% reduction in total laparoscopies, a 3.6% decrease in total laparotomies, and a 4.4% reduction in total surgeries. This led to an overall reduction in total costs of 3.9% (Table 3). Since the ROMA score combines measurements of serum HE4 and CA125, as well as menopausal status into a numerical score, the addition of a second biomarker (HE4), compared to the single biomarker (CA125) measurement already included in the ICRA, led to a 55% increase ($9,177) in total labs costs (ROMA algorithm: $25,865; ICRA: $16,687) (Table 3).
| Table 3: Average lab testing, imaging, and surgery costs for ICRA vs. ROMA in subjects diagnosed with benign disease vs EOC + LMP (n = 441). | |||||||
| Benign vs. EOC + LMP | ROMA | ICRA + ROMA | |||||
| ICRA | ROMA | $ Change | % Change | ICRA + ROMA | $ Change | % Change | |
| Total Labs | $ 16,687 | $ 25,865 | $ 9,177 | 55.0% | $ 25,865 | $ 9,177 | 55.0% |
| Total US | $ 27,626 | $ 27,626 | $ - | 0.0% | $ 27,626 | $ - | 0.0% |
| Total CT Imaging | $ 52,820 | $ 52,820 | $ - | 0.0% | $ 52,820 | $ - | 0.0% |
| Total Laparoscopy | $ 2,082,584 | $ 2,000,065 | $ (82,519) | -4.0% | $ 1,987,066 | $ (95,518) | -4.6% |
| Total Laparotomy | $ 467,198 | $ 452,636 | $ (14,562) | -3.1% | $ 450,342 | $ (16,856) | -3.6% |
| Total Surgery | $ 2,549,782 | $ 2,452,701 | $ (97,081) | -3.8% | $ 2,437,408 | $ (112,374) | -4.4% |
| Total Costs | $ 2,646,915 | $ 2,559,012 | $ (87,904) | -3.3% | $ 2,543,718 | $ (103,197) | -3.9% |
Next, we wanted to expand our analysis to evaluate how the ROMA score may impact healthcare costs for patients with a diagnosis that included all cancers including metastatic disease, non-EOC, and EOC (Table 4). This second cohort included all 461 of the evaluable subjects from the clinical study, 86 of whom were diagnosed with cancer or a low malignant potential tumor (all cancers + LMP). Once again, in this group of subjects, we observed that the ROMA score reduced total healthcare costs by $119,582 (a 4.2% change) when compared to ICRA alone ($2,728,432 vs. $2,848,014). Specifically, it reduced total laparoscopy costs by 4.9%, resulting in a cost saving of $109,799 (ROMA score: $2,135,784, ICRA: $2,245,582), as well as reducing total laparotomy costs by 3.9% ($19,376) (ROMA score: $481,195; ICRA: $500,571). This led to an overall 4.7% reduction in total surgeries, which represents a cost-saving of $129,175. As expected, the combination of ROMA + ICRA in this group of subjects also led to a $ 158,080 (5.6%) reduction in healthcare total costs compared to ICRA alone ($2,689,934 vs. $2,848,014). When looking at the economic impact of ROMA + ICRA on surgeries, the total surgery costs dropped by $167,674 (6.1%) (ICRA alone: $2,746,153; ROMA + ICRA: $2,578,480). Total laparoscopy costs were reduced by 6.3% (ICRA alone: $2,245,582; ROMA + ICRA: $2,103,060) thus leading to a cost change of $142,523, while total laparotomy costs dropped by 5.0% (ICRA alone: $500,571; ROMA + ICRA: $475,420) thus leading to a cost change of $25,151. Overall, these results indicate that the use of the ROMA score, alone or in combination with ICRA, for the triage of women with a pelvic mass does indeed benefit the overall healthcare costs.
| Table 4: Average lab testing, imaging, and surgery costs for ICRA vs. ROMA in subjects diagnosed with benign disease vs all cancers + LMP (n = 461). | |||||||
| Benign vs. All Cancers + LMP | ROMA | ICRA + ROMA | |||||
| ICRA | ROMA | $ Change | % Change | ICRA + ROMA | $ Change | % Change | |
| Total Labs | $ 17,444 | $ 27,038 | $ 9,593 | 55.0% | $ 27,038 | $ 9,593 | 55.0% |
| Total US | $ 28,958 | $ 28,958 | $ - | 0.0% | $ 28,958 | $ - | 0.0% |
| Total CT Imaging | $ 55,458 | $ 55,458 | $ - | 0.0% | $ 55,458 | $ - | 0.0% |
| Total Laparoscopy | $ 2,245,582 | $ 2,135,784 | $ (109,799) | -4.9% | $ 2,103,060 | $ (142,523) | -6.3% |
| Total Laparotomy | $ 500,571 | $ 481,195 | $ (19,376) | -3.9% | $ 475,420 | $ (25,151) | -5.0% |
| Total Surgery | $ 2,746,153 | $ 2,616,978 | $ (129,175) | -4.7% | $ 2,578,480 | $ (167,674) | -6.1% |
| Total Costs | $ 2,848,014 | $ 2,728,432 | $ (119,582) | -4.2% | $ 2,689,934 | $ (158,080) | -5.6% |
Effects on surgeries
Unnecessary procedures, including repeat surgeries, are substantial contributors to cancer care costs. Therefore, we sought to evaluate changes in the numbers of surgeries predicted to be performed by either GYNs or GYN ONCs, as well as the numbers of repeat surgeries, as a readout of using ROMA to assess the risk of malignancy compared to using only ICRA for referral purposes. Specifically, in the group of subjects diagnosed with benign disease vs EOC + LMP (n = 441), 430 subjects received initial or repeated surgery by either a GYN or a GYN ONC. The remaining 11 patients that were evaluated by “Other” physicians (including a cardiovascular surgeon, emergency medicine, gastroenterology, general surgery, medical oncologist, nephrology, nurse midwife, pulmonary/critical care, radiology, reproductive endocrinology & infertility, and urogynecology) were not included in this model projection. Within this sub-population that was evaluated by either a GYN or a GYN ONC, the use of ROMA compared to ICRA for referral purposes predicted a 17% reduction in the total number of surgeries performed by GYNs, possibly due to the increased true positive (TP) rate and the decreased false negative (FN) rate observed with the use of the ROMA score (Table 5). Consequentially, a 64% increase in the number of initial surgeries performed by GYN ONCs was predicted, suggesting once again that the use of the ROMA score instead of ICRA correctly classified more subjects as having a high risk of malignancy after the initial assessment who are then correctly referred to a GYN ONC for surgery. Furthermore, it was predicted that the use of ROMA would lead to a 63% decrease in the number of repeated surgeries performed by GYN ONCs, which can easily be attributed to the observed reduction in FN rates compared to the use of ICRA alone. These results nicely align with the predicted overall 3% reduction in the total number of surgeries performed by both GYNs and GYN ONCs.
| Table 5: Numbers of surgeries predicted to be performed by GYNs and GYN ONCs when using ICRA, ROMA, and ICRA + ROMA for referral purposes in subjects diagnosed with benign disease vs EOC + LMP (n = 430). | |||||
| Surgery performed by: | Predicted Numbers of Surgeries based on referral using: | % Change compared to ICRA Alone for: | |||
| ICRA | ROMA | ICRA + ROMA | ROMA | ICRA + ROMA | |
| GYN (Initial) | 325 | 270 | 239 | -17% | -26% |
| GYN ONC (Initial) | 86 | 141 | 172 | 64% | 100% |
| GYN ONC (Repeat) | 19 | 7 | 6 | -63% | -68% |
| Total # of Surgeries | 430 | 418 | 417 | -3% | -3% |
Similar results were observed when ICRA was used in combination with ROMA. Our model predicted a 26% reduction in the total number of surgeries performed by GYNs, a 100% increase in the total number of surgeries performed by GYN ONC, and a 68% reduction in repeated surgeries performed by GYN ONC for patients assessed by ICRA in combination with ROMA.
We further expanded the analysis to the predicted numbers of surgeries in the benign vs. all cancers + LMP (n = 461) cohort (Table 6). In this cohort, 459 subjects received initial or repeated surgery by either a GYN or a GYN ONC. Within this subpopulation, the analysis results once again confirmed that the use of ROMA was predicted to lead to an 18% reduction in the total number of surgeries performed by GYNs, while increasing by 64% the number of initial surgeries performed by GYN ONCs, thus indicating better patient referral. Use of the ROMA score for referral purposes was also predicted to lead to a 52% decrease in the number of repeated surgeries performed by GYN ONCs, which really represents an impactful economic benefit. Once again, the use of ICRA in combination with ROMA was predicted to lead to a 28% reduction in the total number of surgeries performed by GYN, to double (up to 100%) the number of initial surgeries performed by GYN ONC, while reducing by 69% reduction in the number of repeated surgeries performed by GYN ONC.
| Table 6: Numbers of surgeries predicted to be performed by GYNs and GYN ONCs when using ICRA, ROMA, and ICRA + ROMA for referral purposes in subjects diagnosed with benign disease vs All Cancers + LMP (n = 459). | |||||
| Surgery performed by: | Predicted Numbers of Surgeries based on referral using: | % Change compared to ICRA Alone for: | |||
| ICRA | ROMA | ICRA + ROMA | ROMA | ICRA + ROMA | |
| GYN (Initial) | 336 | 276 | 242 | -18% | -28% |
| GYN ONC (Initial) | 94 | 154 | 188 | 64% | 100% |
| GYN ONC (Repeat) | 29 | 14 | 9 | -52% | -69% |
| Total Surgeries | 459 | 444 | 439 | -3% | -4% |
ICRA assessment stratified by physician specialty
We next sought to evaluate whether physician specialty might contribute to the effectiveness of ICRA to assess women with adnexal masses using the group of subjects diagnosed with benign disease vs EOC + LMP. In this cohort of 441 women with a pelvic mass, 72 were initially assessed by a primary care physician (PCP, which included family medicine, general medicine, hospitalist, internal medicine, med-peds, nurse practitioner, physician assistant, and primary care physician), 339 by a GYN (general obstetrician and gynecologist), and the remaining 30 patients by “Others” (which included cardiovascular surgeon, emergency medicine, gastroenterology, general surgery, medical oncology, nephrology, nurse midwife, pulmonary/critical care, radiology, reproductive endocrinology & infertility, and urogynecology). As observed in Table 7, when comparing ICRA effectiveness based on physician specialty, overall, GYNs were significantly more effective in identifying benign cases when using ICRA compared to PCPs (p value = 0.001) or Others (p value = 0.0006). ICRA performed by GYNs appeared to better identify benign cases in pre-menopausal women compared to PCPs (p value = 0.006) and in post-menopausal women compared to others (p value = 0.037).
| Table 7: The ability of PCPs, GYN, and Others to differentiate between benign and malignant pelvic masses using ICRA in 441 women diagnosed with a benign disease or EOC + LMP. | ||||||
| Benign vs. EOC + LMP | PCP | OB/GYN | Other | |||
| All Patients with a Pelvic Mass | n = 72 | n = 339 | n = 30 | All Patients with a Pelvic Mass | Benign vs. EOC + LMP | |
| True Positives | 19 | 22 | 10 | p values | PCP vs. OB/GYN | OB/GYN vs. Others |
| False Positives | 16 | 36 | 7 | TP vs. FN (Cancer Subjects Only) | 0.350 | 0.237 |
| True Negatives | 33 | 271 | 12 | TN vs. FP (Benign Subjects Only) | 0.001 | 0.006 |
| False Negatives | 4 | 10 | 1 | |||
| Sensitivity | 82.6% | 68.8% | 90.9% | |||
| Specificity | 67.3% | 88.3% | 63.2% | |||
| PPV | 54.3% | 37.9% | 58.8% | |||
| NPV | 89.2% | 96.4% | 92.3% | |||
| FPR | 32.7% | 11.7% | 36.8% | |||
| FNR | 17.4% | 31.3% | 9.1% | |||
| Pre-Menopausal Women Only | n = 25 | n = 199 | n = 12 | Pre-Menopausal Women Only | Benign vs. EOC + LMP | |
| True Positives | 2 | 2 | 3 | p values | PCP vs. OB/GYN | OB/GYN vs. Others |
| False Positives | 6 | 14 | 2 | TP vs. FN (Cancer Subjects Only) | 1.000 | 0.061 |
| True Negatives | 14 | 177 | 7 | TN vs. FP (Benign Subjects Only) | 0.006 | 0.155 |
| False Negatives | 3 | 6 | 0 | |||
| Sensitivity | 40.0% | 25.0% | 100.0% | |||
| Specificity | 70.0% | 92.7% | 77.8% | |||
| PPV | 25.0% | 12.5% | 60.0% | |||
| NPV | 82.4% | 96.7% | 100.0% | |||
| FPR | 30.0% | 7.3% | 22.2% | |||
| FNR | 60.0% | 75.0% | 0.0% | |||
| Post-Menopausal Women Only | n = 47 | n = 140 | n = 18 | Post-Menopausal Women Only | Benign vs. EOC + LMP | |
| True Positives | 17 | 20 | 7 | p values | PCP vs. OB/GYN | OB/GYN vs. Others |
| False Positives | 10 | 22 | 5 | TP vs. FN (Cancer Subjects Only) | 0.371 | 1.000 |
| True Negatives | 19 | 94 | 5 | TN vs. FP (Benign Subjects Only) | 0.083 | 0.037 |
| False Negatives | 1 | 4 | 1 | |||
| Sensitivity | 94.4% | 83.3% | 87.5% | |||
| Specificity | 65.5% | 81.0% | 50.0% | |||
| PPV | 63.0% | 47.6% | 58.3% | |||
| NPV | 95.0% | 95.9% | 83.3% | |||
| FPR | 34.5% | 19.0% | 50.0% | |||
| FNR | 5.6% | 16.7% | 12.5% | |||
We conducted the same analysis using the entire cohort of women who were diagnosed with different histopathological subtypes of cancer (benign vs. all cancers + LMP). In this cohort of 461 women with a pelvic mass, 77 were initially assessed by a PCP, 353 by a GYN and the remaining 31 by “Others”. As observed in Table 8, when comparing ICRA effectiveness based on physician specialty, overall, GYNs were significantly more effective in identifying benign cases when using ICRA compared to PCPs (p value = 0.001) or Others (p value = 0.0006).
| Table 8: The ability of PCPs, GYNs, and Others to differentiate between benign and malignant pelvic masses using ICRA in 461 women diagnosed with a benign disease or all cancers + LMP. | ||||||
| Benign vs. All Cancers + LMP | PCP | GYN | Other | |||
| All Patients with a Pelvic Mass | n = 77 | n = 353 | n = 31 | All Patients with a Pelvic Mass | Benign vs. All Cancers + LMP | |
| True Positives | 23 | 29 | 11 | p values | PCP vs. GYN | GYN vs. Others |
| False Positives | 16 | 36 | 7 | TP vs. FN (Cancer Subjects Only) | 0.116 | 0.081 |
| True Negatives | 33 | 271 | 12 | TN vs. FP (Benign Subjects Only) | 0.001 | 0.006 |
| False Negatives | 5 | 17 | 1 | |||
| Sensitivity | 82.1% | 63.0% | 91.7% | |||
| Specificity | 67.3% | 88.3% | 63.2% | |||
| PPV | 59.0% | 44.6% | 61.1% | |||
| NPV | 86.8% | 94.1% | 92.3% | |||
| FPR | 32.7% | 11.7% | 36.8% | |||
| FNR | 17.9% | 37.0% | 8.3% | |||
| Pre-Menopausal Women Only | n = 25 | n = 203 | n = 12 | Pre-Menopausal Women Only | Benign vs. All Cancers + LMP | |
| True Positives | 2 | 2 | 3 | p values | PCP vs. GYN | GYN vs. Others |
| False Positives | 6 | 14 | 2 | TP vs. FN (Cancer Subjects Only) | 0.538 | 0.022 |
| True Negatives | 14 | 177 | 7 | TN vs. FP (Benign Subjects Only) | 0.006 | 0.155 |
| False Negatives | 3 | 10 | 0 | |||
| Sensitivity | 40.0% | 16.7% | 100.0% | |||
| Specificity | 70.0% | 92.7% | 77.8% | |||
| PPV | 25.0% | 12.5% | 60.0% | |||
| NPV | 82.4% | 94.7% | 100.0% | |||
| FPR | 30.0% | 7.3% | 22.2% | |||
| FNR | 60.0% | 83.3% | 0.0% | |||
| Post-Menopausal Women Only | n = 52 | n = 150 | n = 19 | Post-Menopausal Women Only | Benign vs. All Cancers + LMP | |
| True Positives | 21 | 27 | 8 | p values | PCP vs. GYN | GYN vs. Others |
| False Positives | 10 | 22 | 5 | TP vs. FN (Cancer Subjects Only) | 0.288 | 1.000 |
| True Negatives | 19 | 94 | 5 | TN vs. FP (Benign Subjects Only) | 0.083 | 0.037 |
| False Negatives | 2 | 7 | 1 | |||
| Sensitivity | 91.3% | 79.4% | 88.9% | |||
| Specificity | 65.5% | 81.0% | 50.0% | |||
| PPV | 67.7% | 55.1% | 61.5% | |||
| NPV | 90.5% | 93.1% | 83.3% | |||
| FPR | 34.5% | 19.0% | 50.0% | |||
| FNR | 8.7% | 20.6% | 11.1% | |||
Furthermore, ICRA performed by GYNs appeared to better identify benign cases in pre-menopausal women compared to PCPs (p value = 0.006) and in post-menopausal women compared to others (p value = 0.037). In pre-menopausal women, ICRA performed by GYNs also appeared to better identify cancers when compared to other specialties (p value = 0.022).
Comparison of types of imaging utilized by PCPs and GYNs during ICRA
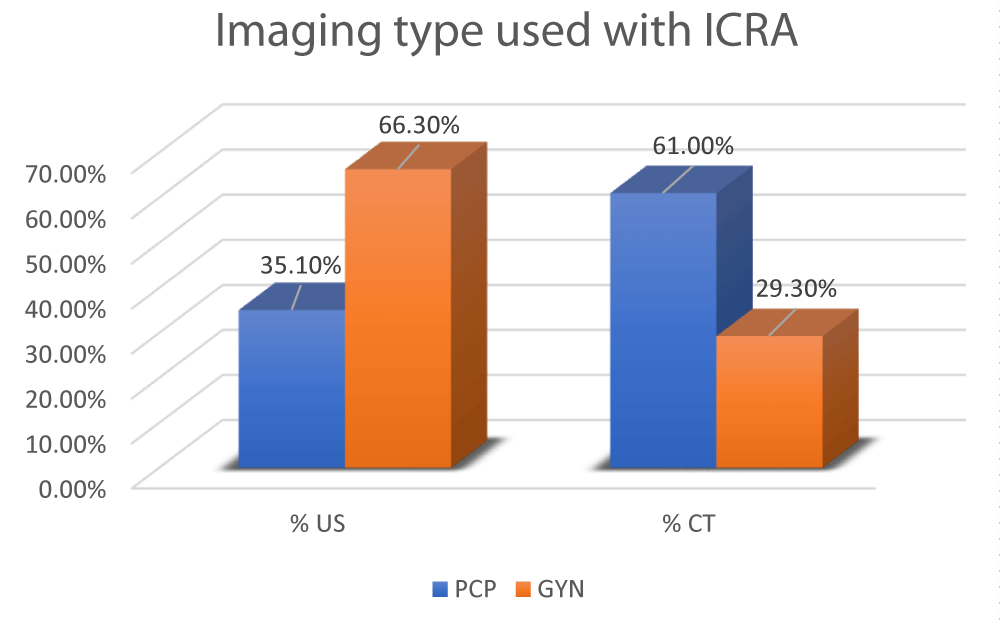
In this study, we sought to determine if physician specialty was linked to different use of imaging (i.e., US vs CT scans) for adnexal mass evaluation, which can contribute to changes in imaging costs. As shown in Figure 3, the type of imaging reported as being used for all evaluable subjects (n = 461) showed that PCPs were 2.1 times more likely to request CT scans when evaluating a pelvic mass compared to GYNs (61.0% vs. 29.3%, respectively). This trend suggests that physicians’ specialty does contribute to differences in imaging costs (Table 9). Specifically, CT scan costs in 100 patients for PCPs would total ~$27,999 compared to ~$13,448 for GYNs, resulting in a price difference of ~$14,551 for every 100 patients (assuming that the imaging was performed in an ambulatory surgical center). On the other hand, ultrasound (US) imaging costs in 100 patients for PCPs would total ~$3,194 compared to ~$6,033 for GYNs, resulting in a price difference of ~$2,839 for every 100 patients (again assuming that the imaging was performed in an ambulatory surgical center).
Figure 3: Type of imaging used with ICRA by PCPs vs. GYNs.
| Table 9: Breakdown of CT scan and US Imaging Costs. | ||||||
| CT-Scan CMS CODE | Ambulatory centers | Patient’s cost | Total cost |
Hospital outpatient | Patient's cost | Total cost |
| 74160 | $125 | $31 | $156 | $197 | $49 | $246 |
| 72193 | $120 | $29 | $149 | $192 | $47 | $239 |
| 71260 | $124 | $30 | $154 | $196 | $48 | $244 |
| Total | $369 | $90 | $459 | $585 | $144 | $729 |
| US Code | Ambulatory centers | Patient’s cost | Total cost | Hospital outpatient | Patient's cost | Total cost |
| 76830 | $73 | $18 | $91 | $117 | $29 | $146 |
Early diagnosis of ovarian cancer, as well as referral of EOC patients to the right level of care, are extremely important for a patient’s survival and quality of life. In fact, the morbidity and survival of ovarian cancer patients are highly dependent on primary surgical and oncological treatment, especially when managed by a gynecologic oncologist [14]. Patients treated by experienced gynecologic oncologists undergo adequate staging in the early stages of the disease and receive a better rate of complete cytoreduction in advanced stages compared to patients treated by general gynecologists [15]. Over the past decade, advancements in medicine and standard of care have allowed for better diagnosis and medical outcomes. The progress in EOC treatment has led to an increase of associated costs. This increase can be partially explained by increased cancer treatment intensity (more patients being treated for longer periods of time), treatment costs, increased use of supportive agents, advanced imaging2, and rising costs of cancer-related surgery and postoperative care [3].
When evaluating the cost of care, the focus should be on potentially modifiable factors that may impact cost without impacting the quality of care [5]. The analysis of cancer care cost is an important step in the evaluation of the economic burden of unnecessary procedures, including repeated surgery, and in the identification of factors contributing to the cost increase. A better understanding of cancer-related costs can help minimize, if not eliminate, unwanted variations in care cost [3]. The main goal of this study was to evaluate the cost-effectiveness of using ICRA, ROMA, or their combination (ICRA + ROMA) for the initial assessment of women with a pelvic mass. As previously mentioned, the use of ICRA alone is often currently used to evaluate women with adnexal masses who are scheduled for surgery. However, contemporary management now includes algorithms like ROMA that include measurements of serum HE4 and CA125 levels (as well as menopausal status), demonstrated improved sensitivity over ICRA alone. Specifically, ROMA allows for a better classification of patients into high and low-risk groups [8] and has higher sensitivity for predicting ovarian cancer in women with a pelvic mass [10]. ROMA has been demonstrated as an accurate tool to effectively triage patients to gynecologic oncologists and centers of excellence for the care of women with ovarian cancer. In this context, the capability of the ROMA score alone or in combination with ICRA to reduce false negative rates in the triage of EOC patients compared to ICRA alone is impactful, as further demonstrated by the present analyses. Namely, patients with adnexal masses that are mistakenly classified at “Low Risk” for harboring a malignancy by the ICRA (due to the high false negative result rate), undergo the initial debulking surgery by a gynecologist rather than by a gynecologic oncologist. Consequently, a false negative result during the initial clinical assessment can significantly impact a patient’s survival and quality of life by 1) delaying the (correct) referral to gynecologic oncologists; 2) delaying cytoreductive surgery and/or treatment [16] and 3) exposing patients to an unnecessary second (repeated) surgery for surgical staging or cytoreduction. Moreover, a correct referral of EOC patients to the appropriate specialist significantly impacts total healthcare costs.
In this study, we saw that the reduction in false negatives due to the introduction of ROMA led to a more appropriate referral of women with malignant adnexal masses to a gynecologic oncologist, as demonstrated by the observed increase in initial surgery rates performed by gynecologic oncologists in both of our cohorts (a 64% increase in both the EOC + LMP cohort and in the All-Cancers cohort). This nicely correlates with the observed reduction in initial surgeries performed by gynecologists (17% and 18% respectively). Furthermore, repeated surgeries also represent a significant economic and social burden. This analysis demonstrated that the introduction of the ROMA score as a triage tool contributed to a reduction in repeated surgeries performed by gynecologic oncologists both inpatients with EOC + LMP patients (63% reduction) and in women with other cancer subtypes (52% reduction). Despite the reduced false negative rates in the triage of both pre-and post-menopausal EOC patients with low malignant potential (LMP) tumors, compared to ICRA, the ROMA score yielded a substantial increase in false positive results (i.e. the classification of a woman with a benign pelvic mass as being “High Risk”). It is important to emphasize that although an increase in false positive rates is associated with additional clinical testing and patient distress, high false negative results (i.e. the classification of a woman with a malignant pelvic mass as being “Low Risk”) represent a more serious concern, since false negatives may prevent women from getting the care they need to treat their cancer.
As previously indicated, imaging also plays a key role in the evaluation and management of adnexal masses. The most common imaging techniques currently utilized in assessing adnexal masses include pelvic ultrasound (US), computed tomography (CT) scans and magnetic resonance imaging (MRI) scans. Pelvic US remains the primary imaging modality used to detect and characterize adnexal masses, followed by CT and/or MRI scanning. As previously indicated in the Medicare 2020 table (and further elucidated in Table 9), costs for US imaging and CT scans appear to be very different. Specifically, the total cost for a transvaginal US (CMS Code 76830) performed in an ambulatory surgical center amounts to ~$91, of which Medicare covers ~$73 (this cost includes facility and doctor fees), and ~$18 is paid by the patient. For the same procedure performed in a hospital outpatient department, the total cost for the transvaginal US amounts to ~$146 (of which ~$117 is covered by Medicare and ~$29 is paid by the patient). Total costs for CT scans (including CMS codes 74160, 72193, and 71260) performed in an ambulatory surgical center amount to ~$459 (of which ~$369 is covered by Medicare and ~$90 is paid by the patient), while the total costs of a CT scan performed in a hospital outpatient department can be as high as ~$729 (of which ~$585 is covered by Medicare and ~$144 is paid by the patient). This represents an approximately 5-fold increase in costs when using CT scans compared to US imaging. In this study we found that PCPs were 2.1 times more likely to request CT scans compared to gynecologists, supporting the idea that imaging testing based on a physician’s specialty does significantly impact costs.
Finally, in this study, we reported that, as expected, lab costs increased by 55% when using the ROMA score compared to ICRA due to the additional detection of a second biomarker (HE4). However, all other costs (including laparoscopy and laparotomy) were significantly decreased when using ROMA alone or in combination with ICRA for the triage of women with a pelvic mass. Specifically, we found that compared to ICRA, the use of ROMA score, alone or in combination with ICRA, led to a reduction in total costs for women with EOC + LMP of 3.3% and 3.9%, respectively. An even greater impact on total costs was observed in the “All Cancers + LMP” cohort, where ROMA alone decreased overall costs by 4.2% and the combination ROMA + ICRA led to an impactful 5.6% reduction in total costs.
To our knowledge, this is the first effort aimed to understand possible variations in costs due to the inclusion of the ROMA algorithm in the initial risk assessment for malignancy in women with ovarian cancer.
This study demonstrated that ROMA’s effectiveness in reducing false negative rates led to a twofold advantage: firstly, a reduction in repeated surgery rates and overall surgeries, leading to better medical outcomes, and secondly, a substantial decrease (3.3%) in the total costs related to ovarian cancer treatment.
Funding source: Fujirebio Diagnostics Inc., Malvern, PA, USA
Highlights
- The economic burden of ovarian cancer is substantial and can depend on many factors including hospitalization, treatment, imaging, and surgeries.
- Correct referral of ovarian cancer patients to the appropriate specialist can significantly reduce healthcare costs.
- The implementation of highly sensitive algorithms in the triage of women with suspected adnexal masses presents a promising approach for reducing healthcare costs associated with ovarian cancer compared to the Initial Clinical Risk Assessment.
Conflict of interest statement
Rianne Esquivel Ph.D.: Is an employee of Fujirebio Diagnostics Inc. Malvern, PA.
Francesca I. DeSimone Ph.D.: Is an employee of Fujirebio Diagnostics Inc. Malvern, PA.
M. Craig Miller: Statistical consultant to Fujirebio Diagnostics Inc. Malvern, PA.
Richard G. Moore, MD: Consultant to Fujirebio Diagnostics Inc. Malvern, PA.
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011 Jan 19;103(2):117-28. doi: 10.1093/jnci/djq495. Epub 2011 Jan 12. Erratum in: J Natl Cancer Inst. 2011 Apr 20;103(8):699. PMID: 21228314; PMCID: PMC3107566.
- Mariotto AB, Enewold L, Zhao J, Zeruto CA, Yabroff KR. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol Biomarkers Prev. 2020 Jul;29(7):1304-1312. doi: 10.1158/1055-9965.EPI-19-1534. Epub 2020 Jun 10. PMID: 32522832.
- Rauh-Hain JA, Hidrue MK, Gaccione P, Melamed A, Meyer LA, Keating NL, Giordano SH, Rice LW, Birrer MJ, Del Carmen MG. Variation in resource utilization associated with the surgical management of ovarian cancer. Gynecol Oncol. 2019 Mar;152(3):587-593. doi: 10.1016/j.ygyno.2018.12.013. Epub 2018 Dec 19. PMID: 30579568; PMCID: PMC6420848.
- Angioli R, Capriglione S, Aloisi A, Miranda A, de Cicco Nardone C, Terranova C, Adrower R, Plotti F. Economic Impact Among Family Caregivers of Patients With Advanced Ovarian Cancer. Int J Gynecol Cancer. 2015 Oct;25(8):1541-6. doi: 10.1097/IGC.0000000000000512. PMID: 26270119.
- Urban RR, He H, Alfonso-Cristancho R, Hardesty MM, Goff BA. The Cost of Initial Care for Medicare Patients With Advanced Ovarian Cancer. J Natl Compr Canc Netw. 2016 Apr;14(4):429-37. doi: 10.6004/jnccn.2016.0049. PMID: 27059191.
- Kwon JS. Cost-effectiveness of Ovarian Cancer Prevention Strategies. Clin Obstet Gynecol. 2017 Dec;60(4):780-788. doi: 10.1097/GRF.0000000000000317. PMID: 28957951.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 174: Evaluation and Management of Adnexal Masses. Obstet Gynecol. 2016 Nov;128(5):e210-e226. doi: 10.1097/AOG.0000000000001768. PMID: 27776072.
- Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, Gajewski W, Kurman R, Bast RC Jr, Skates SJ. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009 Jan;112(1):40-6. doi: 10.1016/j.ygyno.2008.08.031. Epub 2008 Oct 12. PMID: 18851871; PMCID: PMC3594094.
- Nowak M, Janas Ł, Stachowiak G, Stetkiewicz T, Wilczyński JR. Current clinical application of serum biomarkers to detect ovarian cancer. Prz Menopauzalny. 2015 Dec;14(4):254-9. doi: 10.5114/pm.2015.55887. Epub 2015 Nov 27. PMID: 26848298; PMCID: PMC4733894.
- Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, Skates SJ. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-288. doi: 10.1097/AOG.0b013e318224fce2. PMID: 21775843; PMCID: PMC3594110.
- Moore RG, Hawkins DM, Miller MC, Landrum LM, Gajewski W, Ball JJ, Allard WJ, Skates SJ. Combining clinical assessment and the Risk of Ovarian Malignancy Algorithm for the prediction of ovarian cancer. Gynecol Oncol. 2014 Dec;135(3):547-51. doi: 10.1016/j.ygyno.2014.10.017. Epub 2014 Oct 23. PMID: 25449569.
- Rim SH, Hirsch S, Thomas CC, Brewster WR, Cooney D, Thompson TD, Stewart SL. Gynecologic oncologists involvement on ovarian cancer standard of care receipt and survival. World J Obstet Gynecol. 2016;5(2):187-196. doi: 10.5317/wjog.v5.i2.187. Epub 2016 May 10. PMID: 29520338; PMCID: PMC5839163.
- Stewart SL, Cooney D, Hirsch S, Westervelt L, Richards TB, Rim SH, Thomas CC. The Effect of Gynecologic Oncologist Availability on Ovarian Cancer Mortality. World J Obstet Gynecol. 2014 May;3(2):71-77. doi: 10.5317/wjog.v3.i2.71. Epub 2014 May 10. PMID: 26478860; PMCID: PMC4605894.
- Chua KJC, Patel RD, Trivedi R, Greenberg P, Beiter K, Magliaro T, Patel U, Varughese J. Accuracy in Referrals to Gynecologic Oncologists Based on Clinical Presentation for Ovarian Mass. Diagnostics (Basel). 2020 Feb 16;10(2):106. doi: 10.3390/diagnostics10020106. PMID: 32079078; PMCID: PMC7168930.
- Minig L, Padilla-Iserte P, Zorrero C. The Relevance of Gynecologic Oncologists to Provide High-Quality of Care to Women with Gynecological Cancer. Front Oncol. 2016 Jan 14;5:308. doi: 10.3389/fonc.2015.00308. PMID: 26835417; PMCID: PMC4712269.
- U.S. Food & Drug Administration (FDA). Ovarian Cancer Screening Tests: Safety Communication - FDA Recommends Against Use. Published 2016. Accessed December 4, 2021. https://wayback.archive it.org/7993/20180126093611/https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm519540.htm