More Information
Submitted: July 06, 2022 | Approved: July 13, 2022 | Published: July 14, 2022
How to cite this article: Towner M, Underkoffler K, Urh A, Robison K, Moore RG. Patient, disease and surgeon predictors of successful bilateral sentinel lymph node mapping for endometrial cancer: A retrospective, multicenter analysis. Clin J Obstet Gynecol. 2022; 5: 072-079.
DOI: 10.29328/journal.cjog.1001111
Copyright License: © 2022 Towner M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Patient, disease and surgeon predictors of successful bilateral sentinel lymph node mapping for endometrial cancer: A retrospective, multicenter analysis
Mary Towner1,2, Kaylee Underkoffler1, Anze Urh3,4, Katina Robison3 and Richard G Moore1*
1Department of Obstetrics and Gynecology, University of Rochester, Rochester, NY, USA
2Department of Obstetrics and Gynecology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
3Department of Obstetrics and Gynecology, Women and Infants Hospital, Alpert Medical School, Brown University, Providence, RI, USA
4Department of Obstetrics and Gynecology, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hofstra University, East Garden City, NY. USA
*Address for Correspondence: Richard G Moore, MD, Professor, Department of Obstetrics and Gynecology, Chief, Division of Gynecologic Oncology, Wilmot Cancer Center, University of Rochester Medical Center, 125 Lattimore Rd Ste 258, Rochester, NY 14620, USA, Email: [email protected]
Objective: Sentinel lymph node mapping is an acceptable standard for lymph node evaluation in patients with endometrial cancer. The purpose of this study was to evaluate the adoption of this technique at two academic institutions, including which patient and disease features are associated with rates of successfully identifying sentinel lymph nodes with fluorescent mapping. In addition, we sought to characterize if and how surgeons experience the technique related to successful bilateral sentinel lymph node mapping.
Methods: A retrospective chart review was performed of patients at two academic institutions who underwent sentinel lymph node mapping prior to a minimally invasive hysterectomy for endometrial cancer over the first 30 months during which the technique was adopted at each institution. A modified Poisson regression model was used to determine the relationships between patient, disease, and surgeon factors on outcomes of sentinel lymph node mapping.
Results: A total of 460 charts were reviewed. The mean age was 64 and the median body mass index was 34.2. The most disease was stage I (83%), endometrioid (89%), and Grade I (64%). The bilateral sentinel lymph node mapping success rate was 65%, while unilateral or bilateral success occurred in 91% of cases. Sentinel lymph node mapping was significantly more likely to be successful in premenopausal women (RR 1.25; 95% CI 1.07 - 1.46; p = 0.005) and Asian women (RR 1.48; 95% CI 1.3-1.68; p < 0.001). BMI was not significantly predictive of mapping success (RR 1.03; 95% CI 1.00 - 1.07; p = 0.05). Increasing surgeon experience with the technique did predict successful bilateral sentinel lymph node mapping (RR 1.02; 95% CI 1.00 - 1.03; p = 0.02).
Conclusion: Premenopausal status and surgeon experience with the technique increases the likelihood of bilateral sentinel lymph node detection for endometrial cancer.
In 1988, the International Federation of Gynecology and Obstetrics (FIGO) adopted a surgical staging schema for endometrial cancer, which included comprehensive pelvic and para-aortic lymph node dissection [1], though this practice has been debated. Sentinel lymph node (SLN) mapping offers an alternative to comprehensive lymph node dissection in women with endometrial cancer, with several studies that have demonstrated the technique’s high negative predictive value and accuracy [2,3].
It would be reasonable to suspect the success of SLN mapping may be impacted by a variety of patient and disease factors. Prior work has shown increasing BMI, as well as intraoperative lysis of adhesions, to both be negatively associated with detection of SLN [4,5]. Surgeon experience may also play a role. In their 2017 consensus recommendation, the Society of Gynecologic Oncology proposed surgeons adopting the practice of SLN mapping for endometrial cancer continue performing concurrent comprehensive lymph node dissection until their node detection rate meets the literature-comparable rate and their false negative rate reaches < 5% [6]. Khoury-Collado, et al. determined approximately 30 cases might be necessary in order to achieve competency, though the SLN mapping technique used in their analysis is one that is much less commonly used today [7]. No similar study has been done to characterize the relationship between surgeon experience and mapping outcome using modern protocols.
In this retrospective analysis, patient data collected from two academic institutions during the adoption of modern protocols for SLN mapping for endometrial cancer were examined to identify which patient and disease characteristics were associated with failure to detect bilateral SLN during the surgical treatment of endometrial cancer and explore the relationship between surgeon experience and success of the technique.
After receiving approval from the institutional review board at the University of Rochester (project ID: RSRB00072137) and Women’s & Infants Hospital of Rhode Island (project ID: 14 - 0030), a retrospective chart review was performed of all patients over the age of 18 undergoing surgical treatment for endometrial cancer at one of two academic medical centers; data from one center were from 2013 to 2016 and data from the other were from 2015 to 2018. These dates represented the first approximately 30 months during which each institution implemented SLN mapping for endometrial cancer patients. Patients whose surgical plan included laparoscopic (conventional or robotic) SLN mapping and biopsy were included. Patients were identified using pathology reports for which ‘sentinel lymph node’ was included in the specimen label. Patients were excluded if the surgical indication was anything other than endometrial cancer (e.g., cervical cancer).
Surgical protocol
The protocols for sentinel lymph node mapping for endometrial cancer are similar between the two institutions. A total of 1-2 cc of indocyanine green dye was injected into the cervical stroma, divided evenly between the 3 and 9 o’clock positions. Intraoperatively, a near-infrared fluorescence imaging system was used to detect fluorescing lymph nodes. Upon their resection, the lymph nodes were labeled with their laterality and location.
Pathologic protocol
The pathologic ultrastaging process involved preparing a total of 4 slides of 3µm each from the SLN. Slides 1 and 4 were stained with hematoxylin and eosin (H&E) and the adjacent sections undergo immunohistochemistry staining with a pan-cytokeratin cocktail of CAM5.2 (Cell Marque, Rocklin CA) and AE1/AE3 (Dako, Santa Clara CA) antibodies.
Chart review & data analysis
Data collected included patient demographics (e.g., age, race, body mass index (BMI)), disease characteristics (e.g., histologic subtype, depth of invasion, LVSI, grade, stage), and clinical outcomes (e.g., success or failure of SLN detection, number of lymph nodes biopsied, lymph node positivity and location). Additionally, surgical data were collected, including how many cumulative procedures each surgeon had performed at each case timepoint, as well as surgical complications. A database was created using the REDCap electronic data capture tool hosted at the University of Rochester (Harris, Harris). In accordance with the journal’s guidelines, we will provide our data for the reproducibility of this study in other centers if such is requested.
Continuous data were reported as mean or median, as appropriate, while categorical variables were reported as frequency. Chi-square, Wilcoxon rank-sum, and t-tests were used as appropriate to evaluate differences in patient and disease characteristics between groups. For the regression model, BMI was categorized into 5 - number increments (< 20, 20-25, 25 - 30, etc.), in order to allow more clinically relevant interpretations of the results. A surgeon's case number was also categorized into 5-case increments. Poisson regression with robust variance was used to estimate the relative risk of bilateral SLN detection based on patient & disease characteristics, as well as on surgeon experience with the technique. A backward stepwise approach was used to determine appropriate variables for inclusion in the model, with variables yielding a p - value of < 0.2 included in the final regression model. Missing covariate information was low, and so was included in the reference group for each prognostic factor. A marginal effect analysis was done for surgeon case numbers in order to estimate the mean success rate for each case number category. All analyses were conducted in StataBE v.17.
Sentinel lymph node mapping
A total of 460 patient charts were included in the analyses, with 55% receiving treatment at Institution 1 and 45% at Institution 2. Most patients (91%) were non-Hispanic white with a mean age of 63.8. Median BMI was 34.2. Patient demographic and disease characteristics are shown in Table 1. Most tumors were endometrioid subtype (89%), Grade 1 (64%), and Stage I (83%). Of the 460 patients included in the analysis, 9% of patients had their SLN mapping performed laparoscopically (n = 42), with the remainder performed robotically. There was a significant difference in the racial breakdown between the two institutions, as well as histologic subtype distribution. There was a non-significant trend towards patients from Institution 2 tumors having larger tumors that were more likely to be > 50% invasive.
| Table 1: Patient and disease characteristics of patients undergoing SLN dissection for endometrial cancer, by site. | ||||
| Overall (n = 460) | Institution 1 (n = 254) | Institution 2 (n = 206) | P value | |
| Age, mean (SD) | 63.8 (11.1) | 63.6 (11) | 64.5 (11) | 0.99 |
| BMI (kg/m2), median (IQR) | 34.21 (27.8-40.9) | 34.1 (27.6-41) | 34.2 (27.4-40.6) | 0.69 |
| Race, n (%) | 0.02 | |||
| White | 420 (91.3) | 224 (88.2) | 196 (95.1) | |
| Black | 10 (2.2) | 5 (2) | 5 (2.4) | |
| Asian | 6 (1.3) | 4 (1.6) | 2 (1) | |
| Other | 22 (4.8) | 19 (7.5) | 3 (1.5) | |
| Unknown | 2 (0.4) | 2 (0.8) | 0 | |
| Ethnicity, n (%) | 0.63 | |||
| Not Hispanic | 443 (96.3) | 240 (94.5) | 203 (98.5) | |
| Hispanic | 15 (3.3) | 12 (4.7) | 3 (1.5) | |
| Unknown | 2 (0.4) | 2 (0.8) | 0 | |
| Menopause status, n (%) | 0.48 | |||
| Postmenopausal | 405 (88) | 224 (88.2) | 181 (87.9) | |
| Premenopausal | 50 (10.9) | 26 (10.2) | 24 (11.6) | |
| Unknown | 5 (1.1) | 4 (1.6) | 1 (0.5) | |
| Tumor size (mm), median (IQR) | 30 (17-45) | 30 (17-42) | 35 (20-45) | 0.05 |
| Tumor size, n (%) | 0.87 | |||
| ≤ 20 mm | 138 (30) | 77 (30.3) | 61 (29.6) | |
| 20 mm | 322 (70) | 177 (69.7) | 145 (70.4) | |
| Histologic subtype, n (%) | 0.005 | |||
| Endometrioid | 410 (89.1) | 224 (88.2) | 186 (90.3) | |
| Serous | 24 (5.2) | 16 (6.3) | 8 (3.9) | |
| Clear cell | 10 (2.2) | 9 (3.5) | 1 (0.5) | |
| Carcinosarcoma | 7 (1.5) | 3 (1.2) | 4 (1.9) | |
| Mucinous | 2 (0.4) | 2 (0.8) | 0 | |
| Other | 7 (1.5) | 0 | 7 (3.4) | |
| FIGO grade, n (%)a | 0.09 | |||
| Grade 1 | 264 (64.4) | 136 (60.7) | 128 (68.8) | |
| Grade 2 | 109 (26.6) | 61 (27.2) | 48 (25.8) | |
| Grade 3 | 35 (8.5) | 26 (11.6) | 9 (4.8) | |
| Unknown | 2 (0.5) | 1 (0.5) | 1 (0.6) | |
| LVSI, n (%) | 0.92 | |||
| Absent | 359 (78) | 198 (77.9) | 161 (78.2) | |
| Present | 98 (21.3) | 54 (21.3) | 44 (21.3) | |
| Not specified | 3 (0.7) | 2 (0.8) | 1 (0.5) | |
| % myometrial invasion (mm), median (IQR) | 20 (0-53) | 16 (0-50) | 22.2 (0-58) | 0.16 |
| Myometrial invasion, n (%) | 0.02 | |||
| ≤ 50% | 325 (70.7) | 191 (75.2) | 134 (65.1) | |
| >50% | 135 (29.3) | 63 (24.8) | 72 (34.9) | |
| Stage, n (%) | 0.16 | |||
| IA | 311 (67.6) | 178 (70.1) | 133 (64.6) | |
| IB | 72 (15.7) | 29 (11.4) | 43 (20.9) | |
| II | 13 (2.8) | 7 (2.8) | 6 (2.9) | |
| 20 (4.4) | 12 (4.7) | 8 (3.9) | ||
| IIIB | 0 | 0 | 0 | |
| IIIC | 37 (8) | 24 (9.4) | 13 (6.3) | |
| IVA | 0 | 0 | 0 | |
| IVB | 3 (0.6) | 2 (0.8) | 1 (0.5) | |
| Unknown / Not specified | 4 (0.9) | 2 (0.8) | 2 (1) | |
| CI: Confidence Interval; IQR: Interquartile Range; SD: Standard Deviation; a Endometrioid histology (n = 410) | ||||
Bilateral SLN detection was achieved in 65% of cases. At least unilateral detection was achieved in 91% of cases. Table 2 reports patient and disease characteristics by SLN mapping outcome. The only significant difference between groups was disease stage, with more patients having stage IIIC disease in the successful group vs. failure group (p = 0.03). Table 3 shows the unadjusted relative risks of successful bilateral SLN detection based on patient and disease characteristics. In the unadjusted model, characteristics predictive of successful SLN detection were premenopausal status (RR 1.26; 95% CI 1.07 - 1.47; p = 0.004) and Asian race (RR 1.52; 95% CI 1.42 - 1.62; p < 0.001). There was a non-significant trend toward both 5-point increments in BMI (RR 1.04; 95% CI 1.00 - 1.07; p = 0.04) and 5-point increments in the surgeon case number (RR 1.02; 95% CI 1.00 - 1.3; p = 0.02) predicting SLN mapping success. None of the institution, age, surgical approach, tumor size, histology, grade, depth of invasion, presence of lymphovascular space invasion, and stage were associated with successful SLN mapping.
| Table 2: Patient and disease characteristics of patients, by the outcome of SLN mapping technique. | ||||
| Overall (n = 460) | Successful SLN mapping (n = 301) | Failed SLN mapping (n = 159) | P value | |
| Age, mean (SD) | 63.8 (11.1) | 63.5 (10.9) | 64.4 (11.5) | 0.38 |
| BMI (kg/m2), median (IQR) | 34.21 (27.8-40.9) | 34.7 (28.2-41.9) | 33.6 (27-38.5) | 0.06 |
| Race, n (%) | 0.08 | |||
| White | 420 (91.3) | 277 (92) | 143 (90) | |
| Black | 10 (2.2) | 6 (2) | 4 (2.5) | |
| Asian | 6 (1.3) | 6 (2) | 0 | |
| Other | 22 (4.8) | 10 (3.3) | 12 (7.5) | |
| Unknown | 2 (0.4) | 2 (0.6) | 0 | |
| Ethnicity, n (%) | 0.81 | |||
| Not Hispanic | 443 (96.3) | 291 (96.7) | 152 (95.6) | |
| Hispanic | 15 (3.3) | 9 (3) | 6 (3.8) | |
| Unknown | 2 (0.4) | 1 (0.3) | 1 (0.6) | |
| Menopause status, n (%) | 0.07 | |||
| Postmenopausal | 405 (88) | 258 (85.7) | 147 (92.4) | |
| Premenopausal | 50 (10.9) | 40 (13.3) | 10 (6.3) | |
| Unknown | 5 (1.1) | 3 (1) | 2 (1.3) | |
| Tumor size (mm), median (IQR) | 30 (17-45) | 31 (17-45) | 30 (20-44.5) | 0.88 |
| Tumor size, n (%) | 0.95 | |||
| ≤ 20 mm | 138 (30) | 90 (29.9) | 48 (30.2) | |
| 20 mm | 322 (70) | 211 (70.1) | 111 (69.8) | |
| Histologic subtype, n (%) | 0.2 | |||
| Endometrioid | 410 (89.1) | 272 (90.4) | 138 (86.8) | |
| Serous | 24 (5.2) | 13 (4.3) | 11 (7) | |
| Clear cell | 10 (2.2) | 7 (2.3) | 3 (1.9) | |
| Carcinosarcoma | 7 (1.5) | 2 (0.7) | 5 (3.1) | |
| Mucinous | 2 (0.4) | 1 (0.3) | 1 (0.6) | |
| Other | 7 (1.5) | 6 (2) | 1 (0.6) | |
| FIGO grade, n (%)a | 0.21 | |||
| Grade 1 | 264 (64.4) | 166 (61) | 98 (71) | |
| Grade 2 | 109 (26.6) | 80 (29.4) | 29 (21) | |
| Grade 3 | 35 (8.5) | 25 (9.2) | 10 (7.3) | |
| Unknown | 2 (0.5) | 1 (0.4) | 1 (0.7) | |
| LVSI, n (%) | 0.5 | |||
| Absent | 359 (78) | 235 (78.1) | 124 (78) | |
| Present | 98 (21.3) | 65 (21.6) | 33 (20.7) | |
| Not specified | 3 (0.7) | 1 (0.3) | 2 (1.3) | |
| % myometrial invasion (mm), median (IQR) | 20 (0-53) | 20 (0-53) | 16.3 (0-58) | 0.58 |
| Myometrial invasion, n (%) | 0.35 | |||
| ≤ 50% | 325 (70.7) | 217 (72.1) | 108 (67.9) | |
| 50% | 135 (29.3) | 84 (27.9) | 51 (32.1) | |
| Stage, n (%) | 0.03 | |||
| IA | 311 (67.6) | 209 (69.4) | 102 (64.1) | |
| IB | 72 (15.7) | 44 (14.6) | 28 (17.6) | |
| II | 13 (2.8) | 7 (2.4) | 6 (3.8) | |
| IIIA | 20 (4.4) | 9 (3) | 11 (6.9) | |
| IIIB | 0 | 0 | 0 | |
| IIIC | 37 (8) | 30 (10) | 7 (4.4) | |
| IVA | 0 | 0 | 0 | |
| IVB | 3 (0.6) | 1 (0.3) | 2 (1.3) | |
| Unknown/Not specified | 4 (0.9) | 1 (0.3) | 3 (1.9) | |
| CI: Confidence Interval; IQR: Interquartile Range; SD: Standard Deviation; a Endometrioid histology (n = 410) | ||||
| Table 3: Unadjusted risk of successful bilateral SLN mapping. | ||
| Unadjusted Relative Risk (95% CI) | P value | |
| Institution | 0.3 | |
| Institution 1 | Reference | |
| Institution 2 | 1.07 (0.94 - 1.22) | |
| Age | 1.00 (0.99 - 1.00) | 0.39 |
| Race | ||
| White | Reference | |
| Black | 0.91 (0.55 - 1.52) | 0.72 |
| Asian | 1.52 (1.42 - 1.62) | < 0.001 |
| Other | 0.69 (0.43 - 1.09) | 0.12 |
| BMI (5-point increments) | 1.04 (1.00 - 1.07) | 0.04 |
| Premenopausal status | 1.26 (1.07 - 1.47) | 0.004 |
| Laparoscopic approach | 1.02 (0.82 - 1.28) | 0.85 |
| Tumor size > 2 cm | 1.00 (0.87 - 1.16) | 0.95 |
| Non-endometrioid histology | 0.87 (0.68 - 1.12) | 0.28 |
| Grade 3 | 1.08 (0.87 - 1.35) | 0.48 |
| > 50% myometrial invasion | 0.93 (0.8 - 1.09) | 0.36 |
| LVSI | 1.02 (0.87 - 1.21) | 0.79 |
| Stage III (vs Stage I/II) | 1.04 (0.86 - 1.26) | 0.67 |
| Surgeon case number (5-case increments) | 1.02 (1.00 - 1.03) | .02 |
| CI: Confidence Interval | ||
Adjusted relative risks are reported in Table 4. The backward stepwise approach resulted in the inclusion of BMI, menopausal status, race, and surgeon case number in the final model. After adjusting for these covariates, factors significantly predictive of successful SLN mapping were premenopausal status (RR 1.25; 95% CI 1.07 - 1.46; p = 0.005), Asian race (RR 1.48; 95% CI 1.3 - 1.68; p < 0.001), and surgeon case number (RR 1.02; 95% CI 1.00 - 1.03; p = 0.02). Of note, the 95% CI for surgeon case number included 1.00 due to rounding. BMI trended toward predictive, though this did not meet the statistical significance threshold of p < 0.05 (RR 1.03; 95% CI 1.00 - 1.07; p = 0.05).
| Table 4: The adjusted risk of successful bilateral SLN mapping. | ||
| Adjusted Relative Risk (95% CI) | P value | |
| BMI (5 - point increments) | 1.03 (1.00 - 1.07) | 0.05 |
| Menopausal status | ||
| Postmenopausal | 0.8 (0.68 - 0.93) | 0.005 |
| Premenopausal | Reference | |
| Race | ||
| White | Reference | |
| Black | 0.97 (0.58 - 1.62) | 0.91 |
| Asian | 1.48 (1.3 - 1.68) | <0.001 |
| Other | 0.69 (0.44 - 1.09) | 0.11 |
| Surgeon case number (5-case increments) | 1.02 (1.00 - 1.03) | 0.02 |
| CI: Confidence Interval | ||
Surgeon learning
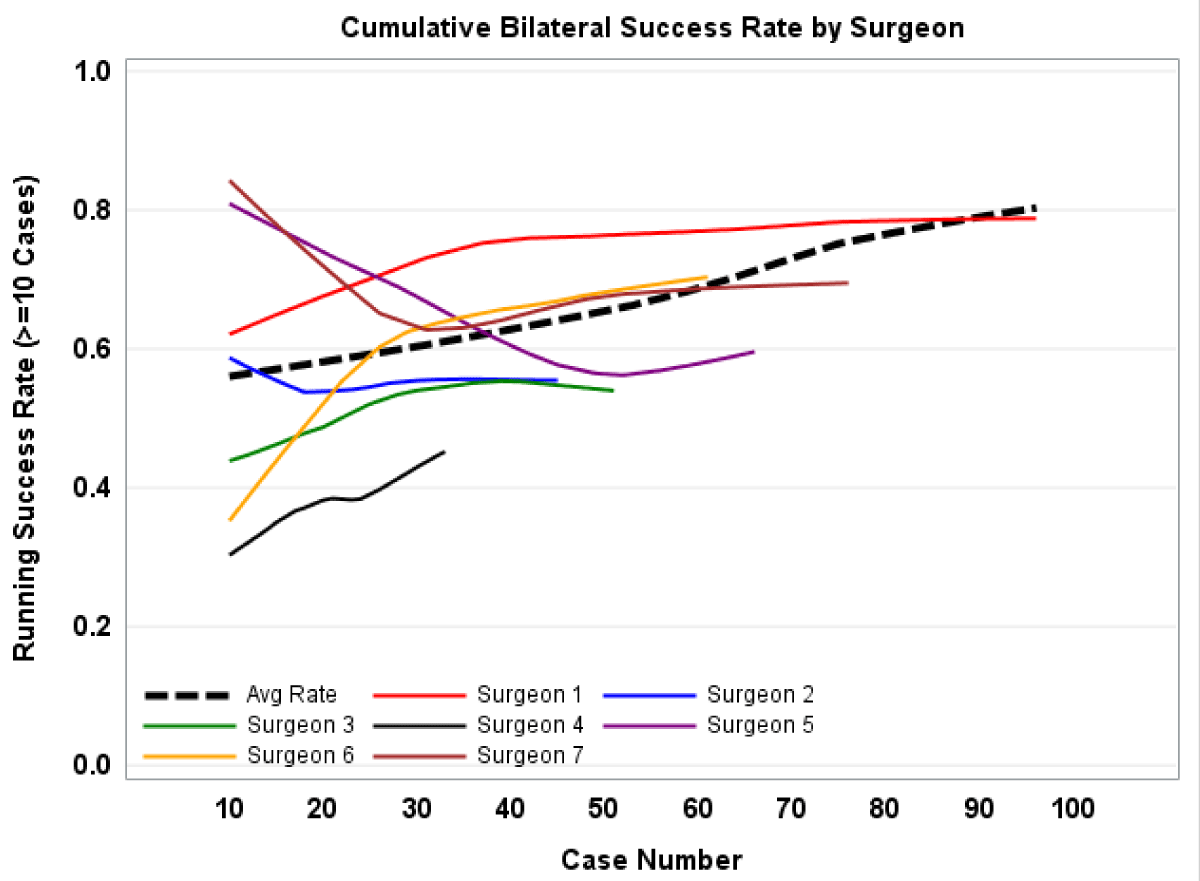
Between the two institutions, 7 surgeons adopted the SLN mapping technique during the study period, five from Institution 1 and three from Institution 2. The number of cases per surgeon ranged from 28 to 97 with a median of 56. There was marked variability in individual surgeon outcomes, with overall bilateral success rates ranging from 45.5% - 78%. There was also variation in how surgeon performance changed with time; four surgeons experienced an improvement in success rates between the first and last 10 cases performed, while one had no change and two had a decrease. Figure 1 depicts the running bilateral SLN mapping success rate, both by the surgeon and overall, with a smoothing effect applied. Using the adjusted regression model reported in Table 4, a marginal effect analysis was performed to determine estimated bilateral SLN mapping success rates based on the surgeon case number. This analysis predicts an improvement in bilateral detection rate from a predicted 58% for the first five cases to a predicted 75% by case 70 (p < 0.001).
Figure 1: Graphical representation of the running bilateral success rate, by surgeon and overall.
Complications
A total of 8 patients had postoperative complications (1.7%). Three (3) of these patients had an unplanned return to the operating room. The remaining complications included (2) perioperative wound infections, (2) vaginal cuff dehiscences, and (1) a new left bundle branch block. There were no perioperative mortalities.
Summary of main results
SLN was successfully mapped bilaterally in 65% of patients. Patient factors predictive of successful SLN mapping were premenopausal status and Asian race, though it should be noted that the number of Asian patients was quite low (n = 6) and we, therefore, hesitate to form any conclusions regarding this finding. There were no differences in SLN mapping outcomes for Black women or those patients categorized as “other” race. Furthermore, we found no previous reports on racial differences in SLN mapping outcomes. Notably, we did not find BMI to be statistically predictive of SLN mapping success.
Surgeon experience with the technique was associated with successful SLN mapping; there was a notable variability in surgeon performance, both at baseline and over the course of technique adoption.
Results in the context of published literature
Endometrial cancer has become the most common gynecologic malignancy in the developed world, directly related to rising rates of obesity [8,9]. Comprehensive surgical staging of endometrial cancer involves pelvic and para-aortic lymphatic dissection, though this practice has been a topic of controversy. Pelvic and para-aortic lymph node dissection is associated with significant morbidity, including an up to 30% risk of lymphedema, and no survival advantage when applied to patients universally [10,11]. Adjuvant treatment in women with the node-positive disease does improve survival over those who do not receive chemotherapy [12], but the likelihood of nodal metastasis at the time of surgical treatment varies widely. Overall, the rate of nodal metastasis for surgically staged endometrial cancer is 6% - 9% [13]. However, patients considered to be low-risk based on the Mayo Criteria of histologic grade, tumor size, and depth of invasion, have a < 1% risk of nodal metastasis, while patients who do not meet the low-risk criteria have a 16% risk [14]. Patients with high-risk histologic features carry up to a 40% nodal metastasis rate [14]. Identification of the tumor features which predict who might benefit from a lymph node dissection relies upon intra-operative frozen pathologic examination, which is not highly accurate and might result in understaging [6].
SLN mapping is considered an acceptable alternative to comprehensive pelvic lymph node dissection for women with endometrial cancer [15]. The technique allows for highly sensitive detection of nodal metastases without subjecting the patient to the same morbidities as a comprehensive nodal dissection. Prior studies reported a cumulative (unilateral or bilateral) success rate of 86% [2] and 89% [16]. Our cumulative success rate was comparable to these, at 91%. However, as the bilateral success rate is the most clinically useful metric, negating the need for further evaluation, we chose it as our primary outcome, rather than including unilateral success. Our data yielded a bilateral success rate of 65%, which is again comparable to the 58% reported by others [16].
Some previous research has suggested there is a lower SLN detection rate in obese women, possibly due to impaired dissemination of the tracer within lymphatic tissue [4,17]. As obesity is common and a direct risk factor for endometrial cancer, this becomes relevant when counseling patients about the possible outcomes of SLN mapping, including failed mapping and the possible need for complete pelvic lymph node dissection. However, more modern trials employing indocyanine green have not found SLN mapping success to be dependent on BMI [18]. We similarly did not find BMI to be predictive of successful SLN mapping. The relationship between BMI and the success rate of SLN mapping is complex, however. When treated as a binary outcome, the association between BMI and SLN mapping success rate became more convoluted. After adjusting for covariates, BMI > 30 had no significant association with SLN mapping success (RR 1.04; 95% CI 0.9 - 1.2; p = 0.57). BMI > 35 was associated with SLN mapping failure (RR 0.78; 95% CI 0.68-0.9; p = 0.001) compared with BMI <35. Meanwhile, BMI > 40 and BMI > 45 were both predictive of SLN mapping success (RR 1.19; 95% CI 1.04-1.36; p = 0.009 & RR 1.21; 95% CI 1.04 - 1.4; p = 0.01, respectively). Perhaps this reflects some inherent difference in women with Class III obesity and further, more detailed investigation into the role obesity plays in sentinel lymph node mapping outcomes is needed. While certain types of obesity may be associated with a higher rate of failed SLN mapping, it is also associated with an earlier stage at diagnosis [19], meaning the likelihood of lymph node status affecting clinical management is low. This may inform decision-making at the time of surgery.
Being postmenopausal was associated with a strong likelihood of failed SLN mapping (RR 0.8; 95% CI 0.68 - 0.94; p = 0.005). With a mean age at the time of endometrial cancer diagnosis of 63, this has implications for patient management, particularly because, in contrast with obesity, increasing age is associated with higher tumor grade [20] and deeper myometrial invasion [20] – the exact factors predicting a higher likelihood of lymph node involvement. Thus, it is extremely pertinent to discuss with older patients the possibility of failed SLN dissection and the acceptable alternatives, bearing in mind these patients may be more likely to have disease features that would warrant adjuvant treatment.
Adopting a new surgical technique might be expected to include some period of adjustment as new skills are acquired. Our findings demonstrate a high degree of variability in surgeon performance during the acquisition of this new skill, with some surgeons improving their success rate and others having stable or even worse performance over time. Nevertheless, our adjusted model suggests surgeon case number is associated with bilateral SLN mapping success, with every 5-case increase in experience being associated with a modest (2%) increase in the chance of success. Our modeled estimate of success rate did not reach a plateau, so we are unable to answer the question of how many cases must be done in order to achieve maximum competency.
Strengths and limitations
Our study included a large sample size from two institutions with a mix of histologic subtypes comparable to other analyses. The outcomes of SLN mapping in our study were similar to those reported by previous authors, supporting the current understanding and application of SLN mapping in endometrial cancer. Collecting data from this particular period also allowed for the unique analysis of surgeon performance of SLN mapping for endometrial cancer during the implementation phase, yielding a better understanding of how the adoption of the technique might impact its success and patient outcome.
Though this study offers both a robust analysis supporting our current understanding of SLN mapping and a new perspective on its implementation, it is not without limitations. Its retrospective nature inherently implies the possibility of errors made during the review of patient charts. It should be noted that the racial breakdown at both institutions does not reflect that seen in other studies or in population-based data on endometrial cancer. Given we had no exclusion criteria related to race, we can only conclude this is related to the catchment population of the institutions; regardless, the lack of minority representation demands caution is used when applying our findings to more general populations.
Another major limitation is not every surgeon routinely performed a concurrent full pelvic LND during their adoption of the technique, making it impossible to calculate a negative predictive value. Further, in addition to the small surgeon sample size, a lack of physician demographics means we were unable to determine what, if any, characteristics (e.g., years of experience) were associated with individual success rates. Furthermore, the variability in surgeon performance makes statistical modeling of the data inherently less reliable. While the model results must be interpreted guardedly, we believe this analysis does provide useful information regarding the implementation of a common surgical technique.
Implications for practice and future research
These findings, in the context of previously published literature, support the notion that increasing experience with SLN mapping increases the success rates of the technique. This emphasizes the importance of surgeons tracking their individual performance, particularly early in the adoption period, to ensure they are meeting literature-reported standard success rates. Patient factors likely impact the chance of SLN success, particularly menopausal status, with postmenopausal status yielding a significantly lower rate of SLN mapping success. This may inform patient counseling, particularly regarding the risk of failed SLN mapping and the need for more extensive LND. Moreover, our study did not find BMI to predict SLN success or failure, perhaps supporting the notion that modern SLN mapping protocols are less impacted by obesity.
This large, multi-institutional analysis demonstrates there are certain patient characteristics that might be predictive of SLN mapping success rates. This information can be used for patient counseling, as well as surgical decision-making, to estimate which patients are more likely to require further lymph node evaluation intraoperatively.
Author contributions
MT was responsible for study conceptualization and methodology, data curation, formal statistical analysis, and manuscript drafting and editing. KU was responsible for data curation and manuscript review and editing. AU was responsible for primary data curation and manuscript review. KR was responsible for primary data curation and manuscript review. RGM was responsible for study conceptualization and methodology, primary data curation, manuscript review, and editing.
We would like to acknowledge Myla Strawderman, for the biostatistical advice she offered during this project.
- Mikuta JJ. International Federation of Gynecology and Obstetrics staging of endometrial cancer 1988. Cancer. 1993 Feb 15;71(4 Suppl):1460-3. doi: 10.1002/cncr.2820710409. PMID: 8431880.
- Rossi EC, Kowalski LD, Scalici J, Cantrell L, Schuler K, Hanna RK, Method M, Ade M, Ivanova A, Boggess JF. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017 Mar;18(3):384-392. doi: 10.1016/S1470-2045(17)30068-2. Epub 2017 Feb 1. PMID: 28159465.
- Bogani G, Murgia F, Ditto A, Raspagliesi F. Sentinel node mapping vs. lymphadenectomy in endometrial cancer: A systematic review and meta-analysis. Gynecol Oncol. 2019 Jun;153(3):676-683. doi: 10.1016/j.ygyno.2019.03.254. Epub 2019 Apr 2. PMID: 30952370.
- Tanner EJ, Sinno AK, Stone RL, Levinson KL, Long KC, Fader AN. Factors associated with successful bilateral sentinel lymph node mapping in endometrial cancer. Gynecol Oncol. 2015 Sep;138(3):542-7. doi: 10.1016/j.ygyno.2015.06.024. Epub 2015 Jun 19. PMID: 26095896.
- Tortorella L, Casarin J, Multinu F, Cappuccio S, McGree ME, Weaver AL, Langstraat CL, Keeney GL, Kumar A, Melis GB, Angioni S, Scambia G, Mariani A, Glaser GE. Sentinel lymph node biopsy with cervical injection of indocyanine green in apparent early-stage endometrial cancer: predictors of unsuccessful mapping. Gynecol Oncol. 2019 Oct;155(1):34-38. doi: 10.1016/j.ygyno.2019.08.008. Epub 2019 Aug 8. PMID: 31402166.
- Holloway RW, Abu-Rustum NR, Backes FJ, Boggess JF, Gotlieb WH, Jeffrey Lowery W, Rossi EC, Tanner EJ, Wolsky RJ. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol Oncol. 2017 Aug;146(2):405-415. doi: 10.1016/j.ygyno.2017.05.027. Epub 2017 May 28. PMID: 28566221; PMCID: PMC6075736.
- Khoury-Collado F, Glaser GE, Zivanovic O, Sonoda Y, Levine DA, Chi DS, Gemignani ML, Barakat RR, Abu-Rustum NR. Improving sentinel lymph node detection rates in endometrial cancer: how many cases are needed? Gynecol Oncol. 2009 Dec;115(3):453-5. doi: 10.1016/j.ygyno.2009.08.026. Epub 2009 Sep 19. PMID: 19767064.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016 Jan-Feb;66(1):7-30. doi: 10.3322/caac.21332. Epub 2016 Jan 7. PMID: 26742998.
- Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016 Mar 12;387(10023):1094-1108. doi: 10.1016/S0140-6736(15)00130-0. Epub 2015 Sep 6. PMID: 26354523.
- Carlson JW, Kauderer J, Hutson A, Carter J, Armer J, Lockwood S, Nolte S, Stewart BR, Wenzel L, Walker J, Fleury A, Bonebrake A, Soper J, Mathews C, Zivanovic O, Richards WE, Tan A, Alberts DS, Barakat RR. GOG 244-The lymphedema and gynecologic cancer (LEG) study: Incidence and risk factors in newly diagnosed patients. Gynecol Oncol. 2020 Feb;156(2):467-474. doi: 10.1016/j.ygyno.2019.10.009. Epub 2019 Dec 16. PMID: 31837831; PMCID: PMC7018616.
- ASTEC study group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009 Jan 10;373(9658):125-36. doi: 10.1016/S0140-6736(08)61766-3. Epub 2008 Dec 16. Erratum in: Lancet. 2009 May 23;373(9677):1764. PMID: 19070889; PMCID: PMC2646126.
- Hogberg T. Adjuvant chemotherapy in endometrial cancer. Int J Gynecol Cancer. 2010 Oct;20(11 Suppl 2):S57-9. doi: 10.1111/IGC.0b013e3181f749fd. PMID: 20975363.
- Rungruang B, Olawaiye AB. Comprehensive surgical staging for endometrial cancer. Rev Obstet Gynecol. 2012;5(1):28-34. PMID: 22582124; PMCID: PMC3349921.
- Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, Podratz KC. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008 Apr;109(1):11-8. doi: 10.1016/j.ygyno.2008.01.023. Epub 2008 Mar 4. PMID: 18304622; PMCID: PMC3667391.
- Hamilton CA, Pothuri B, Arend RC, Backes FJ, Gehrig PA, Soliman PT, Thompson JS, Urban RR, Burke WM. Endometrial cancer: A society of gynecologic oncology evidence-based review and recommendations. Gynecol Oncol. 2021 Mar;160(3):817-826. doi: 10.1016/j.ygyno.2020.12.021. Epub 2021 Jan 27. PMID: 33516529.
- Soliman PT, Westin SN, Dioun S, Sun CC, Euscher E, Munsell MF, Fleming ND, Levenback C, Frumovitz M, Ramirez PT, Lu KH. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecol Oncol. 2017 Aug;146(2):234-239. doi: 10.1016/j.ygyno.2017.05.016. Epub 2017 May 18. PMID: 28528918; PMCID: PMC5860676.
- Vargiu V, Rosati A, Capozzi VA, Sozzi G, Gioè A, Berretta R, Chiantera V, Scambia G, Fanfani F, Cosentino F. Impact of Obesity on Sentinel Lymph Node Mapping in Patients with apparent Early-Stage Endometrial Cancer: The ObeLyX study. Gynecol Oncol. 2022 May;165(2):215-222. doi: 10.1016/j.ygyno.2022.03.003. Epub 2022 Mar 18. PMID: 35314087.
- Sozzi G, Fanfani F, Berretta R, Capozzi VA, Uccella S, Buono N, Giallombardo V, Di Donna MC, Monterossi G, Restaino S, Capasso I, Dinoi G, Scambia G, Chiantera V. Laparoscopic sentinel node mapping with intracervical indocyanine green injection for endometrial cancer: the SENTIFAIL study - a multicentric analysis of predictors of failed mapping. Int J Gynecol Cancer. 2020 Nov;30(11):1713-1718. doi: 10.1136/ijgc-2020-001724. Epub 2020 Aug 31. PMID: 32868384.
- Van Arsdale A, Miller DT, Kuo DY, Isani S, Sanchez L, Nevadunsky NS. Association of obesity with survival in patients with endometrial cancer. Gynecol Oncol. 2019 Jul;154(1):156-162. doi: 10.1016/j.ygyno.2019.03.258. Epub 2019 May 3. PMID: 31060820.
- Mundt AJ, Waggoner S, Yamada D, Rotmensch J, Connell PP. Age as a prognostic factor for recurrence in patients with endometrial carcinoma. Gynecol Oncol. 2000 Oct;79(1):79-85. doi: 10.1006/gyno.2000.5917. PMID: 11006036.