More Information
Submitted: February 07, 2022 | Approved: May 18, 2022 | Published: May 20, 2022
How to cite this article: Elnashar AT, Youssef EM. Immunohistochemical expression of p53 and Fox A1 in epithelial ovarian cancer. Clin J Obstet Gynecol. 2022; 5: 061-066..
DOI: 10.29328/journal.cjog.1001109
Copyright License: © 2022 Elnashar AT, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Ovarian cancer; FOX A1; P53; Prognostic marker; Immunohistochemistry (IHC)
Immunohistochemical expression of p53 and Fox A1 in epithelial ovarian cancer
Afaf T Elnashar1* and Esraa M Youssef2
1Department of Pathology, Faculty of Medicine, Sohag University, Egypt
2Department of Pathology, Faculty of Medicine, Aswan University, Egypt
*Address for Correspondence: Afaf T Elnashar, Department of Pathology, Faculty of Medicine, Sohag University, Egypt, Email: [email protected]
Background: Ovarian cancer (OC) is the fifth cause of cancer mortality in females. There were an estimated 300,000 new cases of OC diagnosed worldwide in 2018, corresponding to 3.4% of all cancer cases among women. The high mortality rate of OC attributed to asymptomatic growth of the tumor leads to its diagnosis at advanced stages. About 85% - 90% of OC are epithelial including serous, endometrioid, clear cell, and mucinous carcinoma.
Aim: To study the immunohistochemical (IHC) expression of FOX A1 and p53 in epithelial OC and its association with prognostic indicators such as age, tumor size, stage, grade, and histological type.
Materials and methods: The study included 52 cases with EOC from the pathology department, faculty of medicine, Aswan, and Sohag Universities, in the period from January 2017 to December 2019. This study involved 52 patients with OC and a median age of 53 years. Different histological types were included as 37 serous, 12 mucinous, 1 case endometroid 2 cases clear cell OC. The study cases were classified into 22 Grade I, 16 Grade II, and 20 Grade III. About 22 cases were at stage I, 9 at stage II, 11 at stage III, and 10 at stage IV. Tissue sections were stained using the IHC technique with FOX A1 at a dilution of 1:100 and p53 at 1:100.
Results: A statistically significant correlation was found between FOX A1 expression and advanced patient's age, high grade, advanced stage, ruptured capsule, and ascites, regardless of tumor laterality. No significant association was found between p53 immunoexpression and the same clinic-pathological parameters although p53 was associated with serious type.
Conclusion: FOX A1 immunoexpression in EOC is considered a poor prognostic factor in EOC. FOX A1 could be a potential therapeutic target and prognostic marker in EOC.
Ovarian cancer (OC) ranks as the fifth leading cause of malignancy-associated mortality in females. There were an estimated 300,000 new cases of ovarian cancer diagnosed worldwide in 2018, corresponding to 3.4% of all cancer cases among women. Although there is substantial geographic variation in the burden of ovarian cancer (rates varying from 5.0 per 100,000 person-years in Africa to 9.5 per 100,000 person/year in Europe). The high mortality rate of OC is attributed to asymptomatic growth of the tumor, delayed onset of symptoms, and lack of proper screening which leads to its diagnosis in the advanced stages. That is why it is called silent killer [1].
Studies showed that up to 90% of all OC have an epithelial origin and the remaining OC are non-epithelial. In all epithelial ovarian cancers (EOCs), 3% are mucinous. There are several histologic types of epithelial ovarian cancer, involving serous, endometrioid, clear cell, mucinous, transitional, and undifferentiated carcinomas [2].
Fork headbox (FOX) A1 represented a potential candidate gene for therapeutic targeting in human EOC; FOX A1 is a transcription factor that is expressed widely and functions in the development of multiple types of human tissue. FOX A1 served a major function in modulating nuclear steroid receptor activity in breast and prostate cancer, and it was suggested that FOX A1 may be associated with pro-tumorigenic phenotypes. FOX A1 is over-expressed in EOC and associated with clinicopathological features, involving overall survival time. FOX A1 potentially represents a novel biomarker and therapeutic target for EOC [3].
P53 is a tumor suppressor protein that regulates the expression of different genes included in apoptosis, growth arrest, inhibition of cell cycle progression, cell differentiation, and DNA repair or senescence in response to genotoxic or cellular stress [4].
Different types of TP53 mutations were observed in 40% - 80% of EOC contributing either to an inactive or a truncated p53 protein. The majority of mutations affecting TP53 are usually located in exons 5–8, coding for the DNA binding domain of the protein. This domain plays a vital role in the activation of the transcription of p53 target genes [5].
The expression of p53 mutants is closely correlated with malignancy and prognosis of EOC, so it could be considered a prognostic indicator [6].
Aim of the work
To study the immunohistochemical (IHC) expression of FOX A1 and p53 in EOC and detect the relationship of their expression with the clinicopathological data of the studied cases, including (age, site of tumor, histological type, grade, stage of tumor, rupture of capsule and ascites).
This perspective and retrospective study was carried out on 52 cases with EOC, selected from archives of the pathology department, faculty of medicine, Aswan, and Sohag Universities in the period from January 2017 to December 2019. The specimens were collected as formalin-fixed, paraffin-embedded tissue blocks. The clinical information of the patients, including age, tumor size, histological type, grade according to World Health Organization (WHO), and stage according to FIGO staging were retrieved. The study was approved by the Ethics Board of Aswan University and informed written consent was taken from every participant in the study. The EOC cases previously-treated with neoadjuvant chemotherapy or radiotherapy and those with incomplete clinical data were excluded. Clinical and pathological data were collected from pathology reports including age, laterality of the tumor, ascites, capsular rupture, stage, histological type, degree of differentiation, and tumor grade were studied. Three serial sections from each tissue block were cut at 4 microns-thickness to be used as follows:
The first section was stained with routine Hematoxylin and Eosin (H&E) to confirm the histological diagnosis and tumor grade. The second section of slides was stained using the IHC technique with rabbit monoclonal antibody against FOX A1, A9793 at a dilution of 1:100. Negative control was run by omitting the primary antibody and positive control for FOX A1 was normal breast tissue. The third slide sections were stained with the rabbit monoclonal antibody against p53; A11232, 1ml concentration with a dilution of 1:100. Positive control for p53 was colorectal carcinoma. All cases were examined using a light microscope. The histopathological type according to the revised World Health Organization Classification of Tumors of the Female Reproductive Organs, were classified into serous, mucinous, endometrioid, and clear cell types [7].
Evaluation of FOX A1 IHC staining
For the evaluation of nuclear IHC staining results, immunoreactivity for FOX A1 in tumor cells was assessed using a scoring system based on staining intensity: negative = 0; 1% - 50% = 1; 51% - 75% = 2; and more than 75% = 3 [8].
Evaluation of p53 IHC staining
Immunoreactivity for p53 was evaluated according to the percentage of positive cells as follows: negative = 0; 1% - 50% = 1; 51% - 75% = 2; and more than 75% = 3 [9].
Statistical analysis of the data
Data were collected, tabulated, and statistically analyzed using Statistical Package for Social Science (SPSS) version 20.0. Qualitative data were described using numbers and percentages. The Kolmogorov-Smirnov test was used to verify the normality of distribution Quantitative data were described using range (minimum and maximum), mean, standard deviation, and median. The significance of the obtained results was judged at the 5% level. The Chi-square test (X²- test) was used to compare qualitative data such as different tumor grades and stages. Fisher’s Exact or Monte Carlo correction: Cor-rection for chi-square when more than 20% of the cells have expected count >5. Student t-test was used in comparing one quantitative variable and one qualitative variable if they were normally distributed. F-test (ANOVA) for normally distributed quantitative variables, to compare between more than two groups. Mann Whitney test for abnormally distributed quantitative variables, to compare two studied groups. Kruskal Wallis test for abnormally distributed quantitative variables, to compare between more than two studied groups. Probability (p - value) difference considered as follow: Statistically significant (S) when (p < 0.05), highly significant (HS) when (p < 0.01) [10,11].
This study involved 52 patients with OC and a median age of 53 years. Different histological types were included as 37 cases (7.2%) of serous, 12 cases (23.1%) mucinous, 1 case (1.9%) endometroid 2 cases (3.8%) clear cell OC. The study cases were classified into 22/52 cases (42.3%) Grade I, 16 cases (34.6%) Grade II, and 20 (48.5%) Grade III as shown in Table 1. Less than half of the cases 22 cases (42.3%) were at stage I, 9 cases (17.3%) at stage II, 11 (21.2%) at stage III, and 10 (19.7%) at stage IV. Bilateral OC was found in 31/52 cases (59.6%) and rupture of the capsule was detected in 30/52 cases (57.7%) while ascites were diagnosed in 29 cases (55.8%) (Table 1).
| Table 1: The clinicopathological data of the studied cases (52 cases). | |||
| The clinicopathological data | No. | % | |
| Age (years) | Min. – Max. | 31:71 | |
| Mean ± SD. | 52.27 ± 11.55 | ||
| Median | 53 y | ||
| Tumour laterality | Unilateral | 21 | 40.40% |
| Bilateral | 31 | 59.60% | |
| Stage | 22 | I | 42.30% |
| 9 | II | 17.30% | |
| 11 | III | 21.20% | |
| 10 | IV | 19.7 | |
| Histological type | 37 | Serous | 71.20% |
| 12 | Mucinous | 23.10% | |
| 1 | Endometrioid | 1.90% | |
| 2 | Clear cell | 3.80% | |
| Grade | 14 | Grade I | 26.90% |
| 18 | Grade II | 34.60% | |
| 20 | Grade III | 38.50% | |
| Capsular rupture | 30 | Yes | 57.70% |
| 22 | No | 42.30% | |
| Ascites | 29 | Yes | 55.80% |
| 23 | No | 44.20% | |
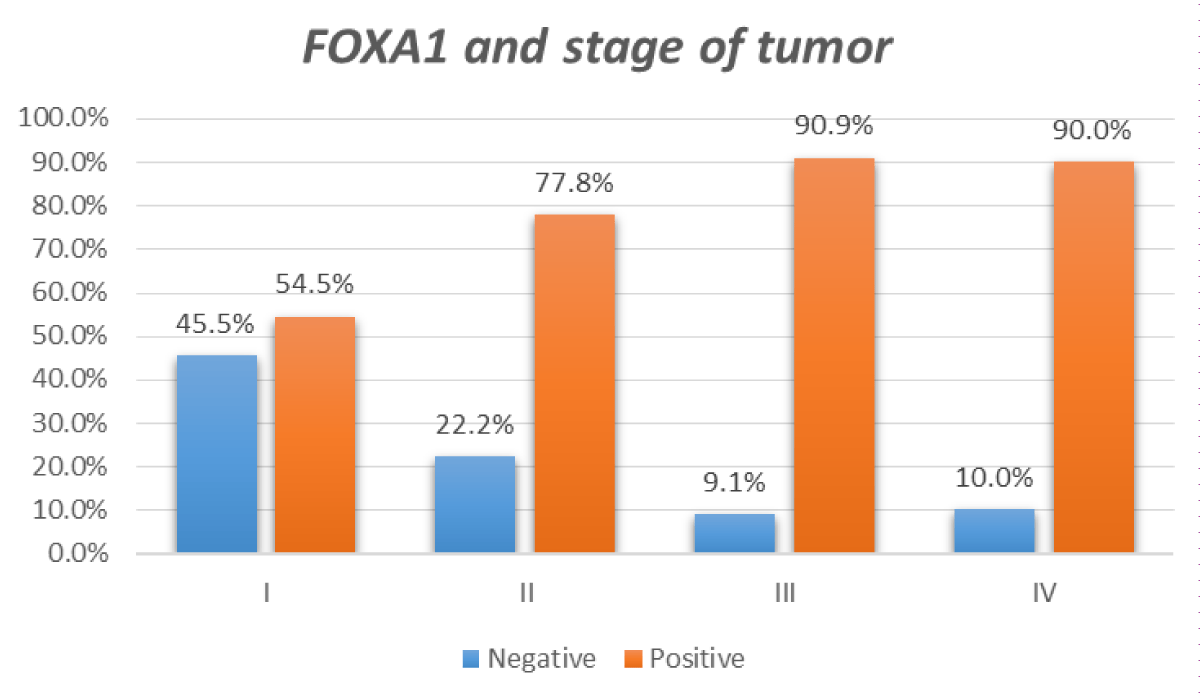
Figure 1: Relation between FOX A1 IHC positivity and stage of tumor.
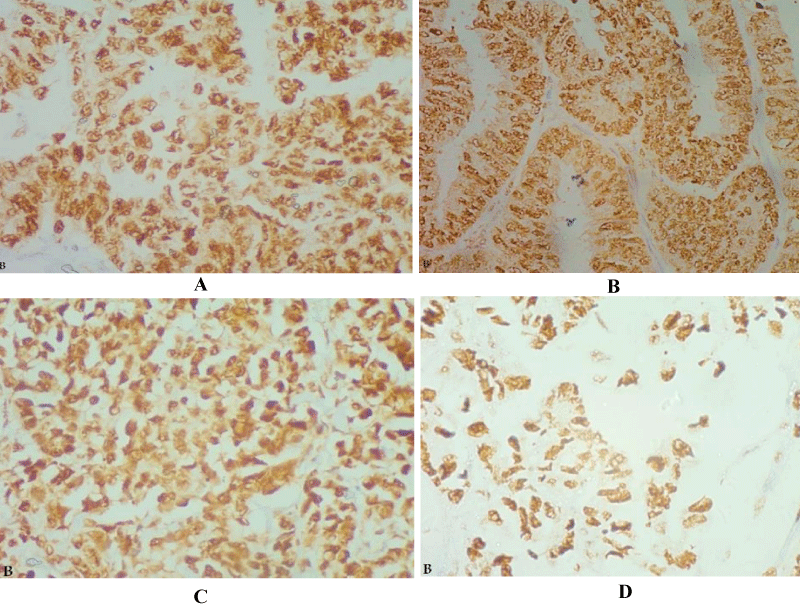
Figure 1a: Serous cystadenocarcinoma, (A) Grade I, (B) Grade II, and (C &D) Grade III, showed strong nuclear FOX A1 expression in tumor cells (IHC X 400).
Assessment of IHC expression of both FOX A1 and p53 was done in malignant epithelial cells for IHC staining status, pattern and intensity. FOX A1 showed a positive nuclear pattern of IHC expression in 38/52 cases (73.1%) and 14/52 (26.9%) were negative (Table 2). According to p53 IHC expression; all positive cases showed a nuclear pattern of expression. 35/52 cases (67.3%) showed positive IHC expression, while 17 cases (32.7%) showed negative expression (Table 3).
| Table 2: Relation between FOX A1 expression clinicopathological features. | |||||
| FOX A1 | |||||
| Positive (n = 38) | Negative (n = 14) | Test of Sig. | p | ||
| No. % | No. % | ||||
| Age (years) | Mean ± SD. | 54.74 ± 10.85 | 45.57 ± 11.04 | t = -2.690 | p = 0.010 |
| Laterality | Unilateral | 14 (66.67%) | 7 (33.33%) | X2 0.736 | p = 0.391 |
| Bilateral | 24 (77.42%) | 7 (22.58) | |||
| Stage | I | 12 (54.5%) | 10 (45.5%) | 0.022 | |
| II | 7 (77%) | 2 (23%) | |||
| III | 10 (90.9%) | 1 (9.1%) | |||
| IV | 9 (90%) | 1 (10.0%) | |||
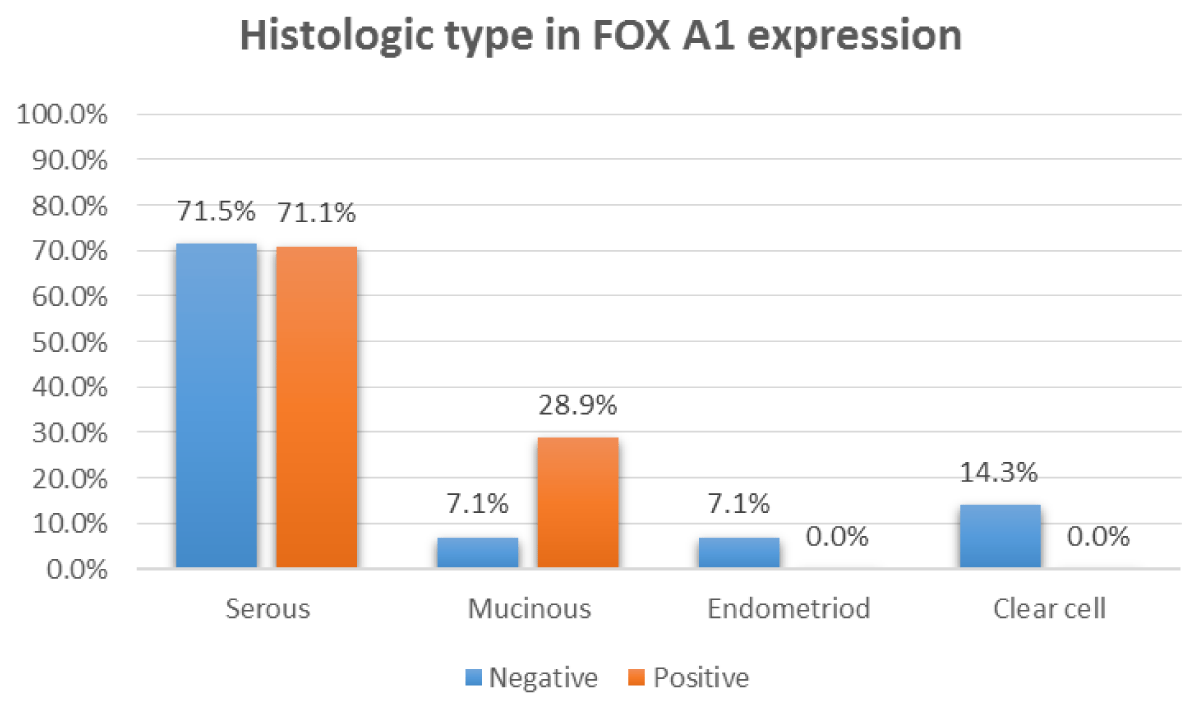
| Histologic type | Serous | 27 (71.1%) | 10 (28.9%) | 0.016 | |
| Mucinous | 11 (28.9%) | 1 (71.1%) | |||
| Endometrioid | - | 1 | |||
| Clear cell | - | 2 | |||
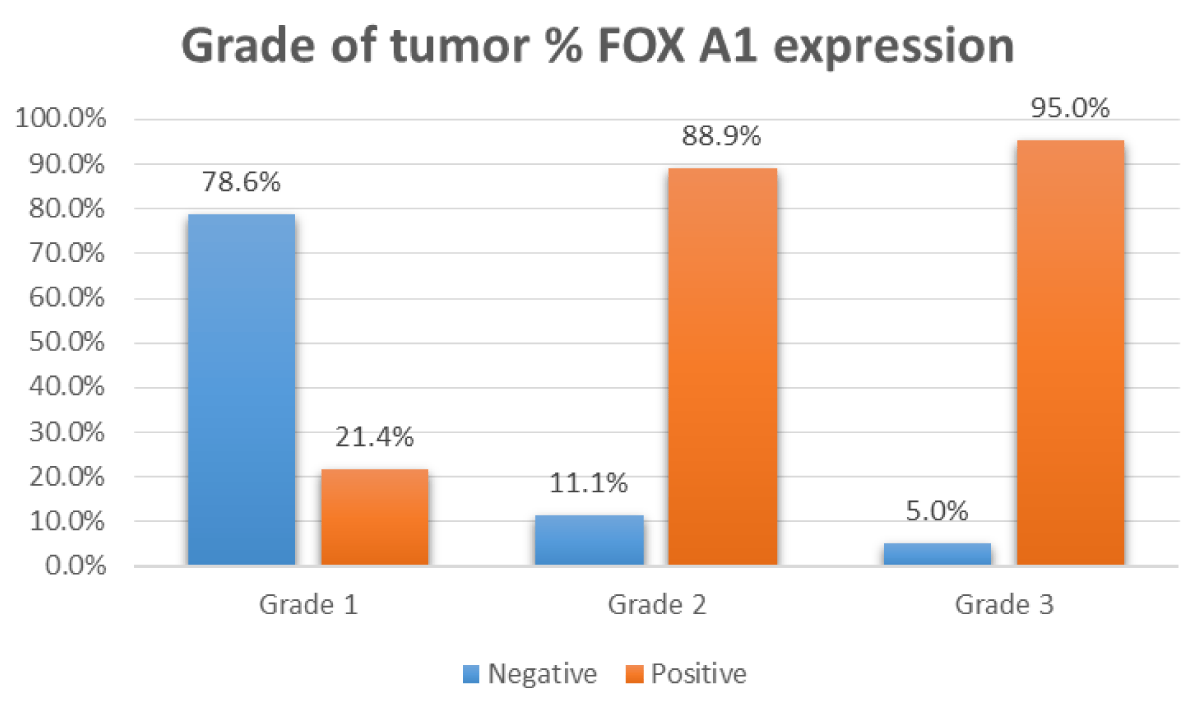
| Grade | Grade I | 3 (21.43%) | 11 (78.57%) | < 0.001 | |
| Grade II | 16 (88.89%) | 2 (11.11%) | |||
| Grade III | 19 (95%) | 1 (5%) | |||
| Capsule rupture | No | 11 (50%) | 11 (50%) | X210.322 | 0.001 |
| Yes | 27 (90%) | 3 (10%) | |||
| Ascites | No | 11 (47.83%) | 12 (52.17%) | X2 13.365 | < 0.001 |
| Yes | 27 (93.1%) | 2 (6.9%) | |||
| c2, p: c2 and p values for Chi-square test FEp: p - value for Fisher Exact for Chi-square test, MCp: p - value for Monte Carlo for Chi-square test t, p: t and p values for Student t - test | |||||
| Table 3: Relation between p53 expression and the clinicopathological features of the studied cases. | |||||
| P53 | Test of Sig | p | |||
| Positive (n = 38) | Negative (n = 14) | ||||
| No.% | No.% | ||||
| Age (years) | Mean ± SD. | 53.69 ± 11.41 | 49.35 ± 11.61 | t = -1.27 | p = 0.207 |
| Laterality | Unilateral | 14 (66.67%) | 7 (33.33%) | X2 0.007 | p = 0.935 |
| Stage | I | 15 | 7 | 0.132 | |
| II | 6 | 3 | |||
| III | 9 | 2 | |||
| IV | 5 | 5 | |||
| Histologic type | Serous | 35 | 2 | < 0.001 | |
| Mucinous | - | 12 | |||
| Endometrioid | - | 1 | |||
| Clear cell | - | 2 | |||
| Grade | Grade I | 9 | 5 | 0.634 | |
| Grade II | 11 | 7 | |||
| Grade III | 15 | 5 | |||
| Capsule rupture | No | 16 | 6 | X2 0.509 | 0.476 |
| yes | 19 | 11 | |||
| Ascites | No | 16 | 7 | X2 0.096 | 0.757 |
| yes | 19 | 10 | |||
| FEp: p - value for Fisher Exact for Chi-square test; MCp: p - value for Monte Carlo for Chi-square test t, p : t and p values for Student t – test. | |||||
Figure 2: Relation between FOX A1 IHC positivity and histologic type.
High significant correlation was found between FOX A1 expression, patient's age (p = 0.010), tumor stage (p = 0.022), histologic type (p = 0.016), tumor grade (p < 0.001), cases with ruptured capsule (p = 0.001) and ascites (p < 0.001) (Table 2).
No significant statistical association could be detected between FOX A1 expression and tumor site (unilateral or bilateral) in the studied cases (Table 2).
There was a high statistically significant association between p53 expression and histological type of the tumor (p < 0.001) (Table 3).
No significant correlation could be detected between age, tumor site (unilateral or bilateral), tumor grade, tumor stage, the status of the capsule (intact or reputed), ascites, and p53 expression (Table 3).
Figure 3: Relation between FOX A1 IHC positivity and grade of the tumor.
Ovarian cancer (OC) is the second most common type of gynecological cancer worldwide, and it is a major cause of cancer-associated mortality in women [13]. There were an estimated 300,000 new ovarian cancer cases diagnosed worldwide in 2018 which was corresponding to 3.4% of all cancer cases among women. However, there is a substantial geographic variation in the burden of OC (rates are varying from 5.0/100,000 person/year in Africa to 9.5/100,000 person/year in Europe) [14].
The more practically accepted concept of classification of OC is into 5 main histological types as follows: high-grade serous carcinoma (HGSC), clear-cell carcinoma, endometrioid carcinoma, mucinous carcinoma, and low-grade serous carcinoma (LGSC). This classification depends on differences in their biology, clinical presentation, and response to chemotherapy [15].
The current study aimed to evaluate the IHC expression of FOX A1 and p53 in tumor cells and their effect on the prognosis of EOC.
In this study, cases were patients of ages ranging from 31- 71 years. The mean age of the cases was 52.27 years, while the median age was 53 years. The age of the patients in this study was comparable to those reported by Wang, et al. who studied 110 cases of primary EOC and reported that the mean age of the patients was 54.5 years (range, 29–78 years) [16].
In the present work, bilateral tumors were the most frequent (59.6%). This result was comparable to those reported by Wang, et al. as they reported that 46.4% of tumors were bilateral [16].
For histologic type, the current study denoted that more than (70%) of studied cases were serous carcinoma. Wang, et al., reported that 40% of tumors were HGSC. Also, Amanullah, et al., reported that 48.3% were serious tumors [16,17].
Regarding tumor grade, high-grade tumors were found to represent the highest percentage 73.0% of cases in the current research. This is in agreement with a study done by Ndukwe, et al., who reported that (66%) of tumors were high-grade neoplasms and (34%) of the case were low-grade neoplasms [18].
The state of the capsule and the presence of ascites are powerful prognostic factors and are routinely used to determine the stage of the tumor. In the current study, 57.7% of cases had ruptured capsules and 55.8% of cases had ascites, compared with 73% of cases that had ruptured capsules in the study of Amanullah, et al. [17].
In the current study, p53 expression using IHC staining was detected in (67.3%) of the studied cases. A previous study was done by Amanullah, et al., which reported p53 expression in (65.2%) of EOC samples. Furthermore, p53 positivity was detected in (58%) of EOC specimens according to a study carried out by Ndukwe, et al. on 50 specimens of EOC tissues. In contrast, a study carried out by Mohamed et al., showed a lower percentage (35.1%) of p53 expression in EOC [17-19].
In the current study, all p53-positive cases were serious malignancies. Malignant mucinous tumors, clear cell tumors, and endometrioid carcinoma cases were p53 negative. These results agree with Zhang, et al. who found strong p53 expression in (70.8%) of serous carcinomas and showed that the tumor histologic type was closely associated with the expression of p53 [20].
According to the relation between p53 expression and other clinicopathological variables; no significant association could be detected between p53 expression in tumor cells and other clinic-pathologic parameters including age, laterality, stage, state of the capsule, and presence or absence of ascites. This study showed no significant statistical relation between p53 expression and tumor grade in contrast to Ndukwe, et al. who had reported that p53 immunopositivity was significantly associated with high-grade tumors in general and high-grade serous carcinomas in particular [18].
Regarding Immunohistochemical expression of FOX A1 in EOC specimens; the present study demonstrated increased expression of FOX A1 in EOC tissues (73.1%). A study that was carried out by Wang, et al., reported that FOX A1 expression was seen in about 73.6% of EOC tissues [16].
According to the relation between FOX A1 expression and tumor stage; FOX A1 was positive in 90.48% of cases at stages III and IV. This result agreed with Zhang, et al. who detected FOX A1 in 84.7% of cases at stages III and IV [20].
In this study, a significant statistical relation could be detected between FOX A1 expression and tumor grade as 92.1% of tumors of grade II and grade III showed positive expression of FOX A1. This was concomitant with the study of Wang, et al. who observed that 41/62 cases of moderately differentiated and poorly differentiated EOC showed strong FOX A1 expression of tumor cells and that was statistically significant. These findings suggest that FOX A1 expression was associated with poor prognostic parameters in EOC [16].
In the current work; (71.1%) of positive FOX A1 expression cases were serous carcinomas of which (66.7%) and showed a significant relation between FOX A1 expression and histologic type of tumor. These results disagreed with Wang, et al. who reported that only 55% of serous carcinomas were stained positive with FOX A1. This discrepancy between results may be attributed to the difference in sample size [16].
According to the current work, there was a highly significant association between FOX A1 expression and tumors with ruptured capsules and ascites.
In conclusion, FOX A1 immunoexpression in EOC is considered a poor prognostic parameter as it is expressed in the majority of EOC, especially with advanced age, high grade, poor differentiation, advanced stages, tumors with ruptured capsule and ascites regardless of the side of the tumor (unilateral or bilateral). While p53 immunopositivity is significantly associated with the majority of cases of serous carcinoma, no significant association could be found between p53 immunoexpressing and other clinic-pathological parameters such as age, size of the tumor, tumor grade, stage, state of the capsule, and ascites. The results of the present study indicated that FOX A1 could serve an important function in EOC and may be a potential therapeutic target and prognostic marker.
- Yoneda A, Lendorf ME, Couchman JR, Multhaupt HA. Breast and ovarian cancers: a survey and possible roles for the cell surface heparan sulfate proteoglycans. J Histochem Cytochem. 2012 Jan;60(1):9-21. doi: 10.1369/0022155411428469. PMID: 22205677; PMCID: PMC3283135.
- Chang HT, Chiu ML, Wang TY, Chen TC, Chang CL, Su TH, Wang KG, Wang KL, Yang YC, Chen JR. Effect of Chemotherapy, Laparoscopy, and Cytology on Stage IC Ovarian Clear Cell Carcinoma: A Long-Term, Single-Center Study. Int J Environ Res Public Health. 2020 Jan 13;17(2):491. doi: 10.3390/ijerph17020491. PMID: 31940991; PMCID: PMC7014224.
- Wang K, Guan C, Fang C, Jin X, Yu J, Zhang Y, Zheng L. Clinical significance and prognostic value of Forkhead box A1 expression in human epithelial ovarian cancer. Oncol Lett. 2018 Apr;15(4):4457-4462. doi: 10.3892/ol.2018.7899. Epub 2018 Jan 29. PMID: 29541214; PMCID: PMC5835846.
- Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011 May-Jun;61(3):183-203. doi: 10.3322/caac.20113. Epub 2011 Apr 26. PMID: 21521830; PMCID: PMC3576854.
- Antoun S, Atallah D, Tahtouh R, Alaaeddine N, Moubarak M, Khaddage A, Ayoub EN, Chahine G, Hilal G. Different TP53 mutants in p53 overexpressed epithelial ovarian carcinoma can be associated both with altered and unaltered glycolytic and apoptotic profiles. Cancer Cell Int. 2018 Jan 30;18:14. doi: 10.1186/s12935-018-0514-2. PMID: 29422776; PMCID: PMC5791177.
- Chen L, Li L, Chen F, He D. Immunoexpression and prognostic role of p53 in different subtypes of epithelial ovarian carcinoma. J Biomed Res. 2012 Jul;26(4):274-7. doi: 10.7555/JBR.26.20110103. Epub 2012 Apr 24. PMID: 23554760; PMCID: PMC3596744.
- Meinhold-Heerlein I, Fotopoulou C, Harter P, Kurzeder C, Mustea A, Wimberger P, Hauptmann S, Sehouli J. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch Gynecol Obstet. 2016 Apr;293(4):695-700. doi: 10.1007/s00404-016-4035-8. Epub 2016 Feb 19. Erratum in: Arch Gynecol Obstet. 2016 Jun;293(6):1367. PMID: 26894303.
- Wang LL, Xiu YL, Chen X, Sun KX, Chen S, Wu DD, Liu BL, Zhao Y. The transcription factor FOX A1 induces epithelial ovarian cancer tumorigenesis and progression. Tumour Biol. 2017 May;39(5):1010428317706210. doi: 10.1177/1010428317706210. PMID: 28488543.
- Günakan E, Tohma YA, Karakaş LA, Akıllı H, Haberal AN, Ayhan A. Prognostic impact of p16 and p53 gene expressions in stage 1a epithelial ovarian cancer. Obstet Gynecol Sci. 2020 Jul;63(4):464-469. doi: 10.5468/ogs.19204. Epub 2020 Jun 19. PMID: 32550735; PMCID: PMC7393742.
- Kirkpatrick LA. (Bundle: A Simple Guide to IBM SPSS Statistics - version 23.0, 14th + IBM SPSS Statistics Student Version 21.0 for Windows 14th Edition
- Hånell A. Discovery reliability. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2019;39(6): 1185–1187.
- Vargas AN. Natural history of ovarian cancer. J Cancer Sci Ther. 2014;6:247–52.
- Peres LC, Cushing-Haugen KL, Köbel M, Harris HR, Berchuck A, Rossing MA, Schildkraut JM, Doherty JA. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J Natl Cancer Inst. 2019 Jan 1;111(1):60-68. doi: 10.1093/jnci/djy071. PMID: 29718305; PMCID: PMC6335112.
- Cabasag CJ, Arnold M, Butler J, Inoue M, Trabert B, Webb PM, Bray F, Soerjomataram I. The influence of birth cohort and calendar period on global trends in ovarian cancer incidence. Int J Cancer. 2020 Feb 1;146(3):749-758. doi: 10.1002/ijc.32322. Epub 2019 Apr 30. PMID: 30968402; PMCID: PMC6786921.
- Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012 Mar;460(3):237-49. doi: 10.1007/s00428-012-1203-5. Epub 2012 Feb 10. PMID: 22322322.
- Wang K, Guan C, Fang C, Jin X, Yu J, Zhang Y, Zheng L. Clinical significance and prognostic value of Forkhead box A1 expression in human epithelial ovarian cancer. Oncol Lett. 2018 Apr;15(4):4457-4462. doi: 10.3892/ol.2018.7899. Epub 2018 Jan 29. PMID: 29541214; PMCID: PMC5835846.
- Razak Amanullah NA, Poothiode U, Vilasiniamma L. Expression of p53 in epithelial ovarian tumors. Indian J Pathol Microbiol. 2020 Apr-Jun;63(2):235-240. doi: 10.4103/IJPM.IJPM_526_19. PMID: 32317522.
- Ndukwe CO, Azuoma LA, Onyiaorah IV. Profile of p53 expression in EOC: A multicenter study from South-East Nigeria. CCIJ. 2018; 10.4103.
- Mohamed AO, Husain NE, Elmassry ER, Alnageeb L, Elhassan M, Abdelaziz MS. IHC expression of p53 in Type I and II EOC among Sudanese women: a cross-sectional study; F1000 Research. 2019; 8:1739.
- Zhang G, Zhao Y, Liu Y, Kao LP, Wang X, Skerry B, Li Z. FOX A1 defines cancer cell specificity. Sci Adv. 2016 Mar 18;2(3):e1501473. doi: 10.1126/sciadv.1501473. PMID: 27034986; PMCID: PMC4803482.