More Information
Submitted: February 23, 2022 | Approved: March 10, 2022 | Published: March 11, 2022
How to cite this article: Lecourt A, Labrosse J, Peigné M, Vinolas C, Laup L, et al. Addition of dydrogesterone to vaginal progesterone and transfer postponement improve outcomes in patients with low progesterone levels in hormonally substituted cycles for frozen-thawed embryo transfer. Clin J Obstet Gynecol. 2022; 5: 027-035.
DOI: 10.29328/journal.cjog.1001103
Copyright License: © 2022 Lecourt A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Frozen thawed embryo transfer; Hormone replacement treatment; Serum progesterone; Dydrogesterone; Live birth
Addition of dydrogesterone to vaginal progesterone and transfer postponement improve outcomes in patients with low progesterone levels in hormonally substituted cycles for frozen-thawed embryo transfer
Anne Lecourt1, Julie Labrosse1, Maeliss Peigné1,2, Claire Vinolas1, Laetitia Laup1, Christophe Sifer3, Michael Grynberg1,2 and Isabelle Cedrin-Durnerin1*
1AP-HP Service de Médecine de la Reproduction et Préservation de la Fertilité, Hôpital Jean-Verdier, Avenue du 14 Juillet, Bondy 93140, France
2Université Sorbonne Paris Nord, Bobigny, France
3AP-HP Service de Biologie de la Reproduction, d’Histo-Embryologie et Cytogénétique, Hôpital Jean-Verdier, Avenue du 14 Juillet, Bondy 93140, France
*Address for Correspondence: Dr. Isabelle Cedrin-Durnerin, Hôpital Jean-Verdier, Avenue du 14 Juillet, Bondy 93140, France, Email: [email protected]
Purpose: Adding dydrogesterone (DYD) to vaginal micronized progesterone (VMP) and postponing embryo transfer in order to improve outcomes in patients with low progesterone (P) levels in hormonally substituted cycles prior to frozen/thawed embryo transfer (FET).
Methods: Endometrial preparation comprised sequential administration of vaginal estradiol until endometrial thickness reached 7 mm, followed by transdermal estradiol combined with 800 mg/day VMP. Our previous analysis of serum P levels on FET day showed that the optimal P level was > 11 ng/mL for live birth. Serum P was measured on day 1 (D1) following exogenous VMP introduction in the evening. When P levels were > 11 ng/mL, FET was performed “in phase” on day-2, day-3, or day-5 depending on embryo stage at cryopreservation (n = 139 cycles). When P levels were < 11 ng/mL, DYD 10 mg three times a day orally, was added to VMP and FET was postponed by one day (n = 237 cycles, 63%). The primary endpoint was the comparison of live birth rates (LBR) between the two groups.
Results: Mean serum P level on D1 was 10.2 + 3.7 ng/mL. Characteristics of patients in both groups were similar for age, body mass index, endometrial thickness prior to P introduction, quality of transferred embryos, and embryo transfer stage. Regarding the primary endpoint, LBR was similar between the VMP+DYD group and the VMP group (26.1% vs. 27.3%, NS).
Conclusion: These results suggest that adding DYD to VMP and postponing the transfer in patients with low P levels in hormonally substituted FET cycles might optimize outcomes.
Over the past decade, frozen embryo transfer (FET) has become the most prevalent procedure in modern assisted reproductive techniques (ART). This evolution is related to several concomitant factors: i) the development of the vitrification technique for embryo cryopreservation with increased survival rates [1] and reassuring safety data [2], ii) introduction of elective single embryo transfer policy in most ART centers, iii) raising strategy of freeze-all to prevent ovarian hyperstimulation syndrome and to avoid adverse effects of ovarian stimulation on endometrial receptivity [3] and iv) increasing popularity of preimplantation genetic testing.
Currently, no consensus exists on the optimal method of endometrial preparation prior to FET in normal-ovulatory patients. Indeed, no difference was reported in terms of clinical pregnancy rates or live birth rates (LBR) between endometrial preparation by natural cycle, mildly stimulated cycle, or hormonal replacement therapy (HRT) [4–6]. However, endometrial preparation with HRT is the most used worldwide since it enables centers to schedule FET more conveniently. Nevertheless, the most recent Cochrane systematic review showed low-quality evidence that LBR might be decreased in artificial cycles compared to stimulated cycles [7]. This suggests that there is room for improvement in endometrial preparation to ensure an optimal synchroni-zation between endometrium and embryo.
In HRT cycles, endometrial receptivity seems to be related to both the duration and the dose of P exposure after adequate estrogen impregnation. Exogenous P can be administered orally, vaginally, rectally, subcutaneously, or intramuscularly. The vaginal route of P administration appears to be the method of choice in terms of effectiveness and side effects compared to the intramuscular route [8–11]. Furthermore, uterine P concentrations are ten times higher than serum levels using the vaginal route, known as the “first uterine pass” [12]. Finally, vaginal administration allows for rapid P absorption in a few hours and achievement of serum steady state within 24 hours [13-15]. Nevertheless, great inter-individual variability is constantly reported and this discrepancy might be the consequence of variations in vaginal absorption or bioavailability.
Although the monitoring of serum P levels is not performed in routine practice in FET cycles with vaginal P administration, recent evidence shows that serum P levels have an impact on outcomes. Indeed, it has been reported that low P levels around the day of embryo transfer [16–21] or on the day of pregnancy test [22–24] and in fact, all across the luteal phase [25], were associated with significantly decreased ongoing pregnancy or LBR. Currently, the optimal serum P threshold is not consensual. The minimal optimal serum P - value following vaginal administration of P seems to be between 8.8 and 15 ng/mL. According to these studies, P levels below the minimal threshold effect from a quarter up to half of the patients receiving vaginal P administration and seems to be a reproducible phenomenon from one cycle to another in a single patient [26,27]. On the other hand, a P ceiling not to surpass optimal results was reported only with high doses of vaginal P [16] or combined vaginal and rectal routes of P administration [28].
Despite the established negative impact of low P levels on outcomes in FET, the optimal threshold and timing of P measurement and the different treatment strategies to overcome this issue in case of low P levels remain to be determined. In our first study [19], increasing the vaginal P dose to 1200 mg/day if P levels were below 10 ng/mL at the time of transfer seemed ineffective to improve outcomes. Conversely, adding P via SC or IM route on the day of blastocyst transfer or the day before has been tested successfully [29-31].
This discrepancy could arise from the limited absorption of P by the vaginal route while the parenteral route quickly increases serum P concentrations to high levels. As the daily IM route of P was not available in our country and the SC route was at the financial charge of patients, another alternative was the adjunction of oral P in the form of dydrogesterone (DYD). DYD is an oral retroprogesterone, a selective P receptor agonist, having a lower affinity for androgen and glucocorticoid receptors [32] and a better oral bioavailability compared with oral micronized P [33]. Although it was shown to be non-inferior to vaginal P at the dose of 30 mg/day in fresh embryo transfers [34–38], limited data were available concerning its use in HRT FET cycles [39,40].
Under the assumption that an optimal P window, characterized by minimum P levels during a certain time, is necessary to optimize embryo implantation, we tried to measure P as sooner as possible, i.e. one day after initiation of treatment according to previously described pharmacokinetic studies. In our experience of serum P measurement in HRT cycles, we indeed observed similar mean serum P levels measured on day 2 of VMP administration in a mock cycle prior to oocyte donation [27] and on the ET day in FET cycles [19]. Therefore, our routine HRT protocol was modified by measuring P levels the day after initiation of VMP in order to adjust P treatment and timing of ET in patients according to measured P level. In patients with normal P levels, VMP was continued at the same dose and FET was performed “in phase” depending on embryo stage at cryopreservation. In patients with low P levels, DYD was added to VMP and embryo transfer was postponed by one day. The aim of the present study was to determine if this new protocol improved outcomes in patients with low P levels using HRT for endometrial preparation prior to FET.
Patients
This study was conducted in the Department of Reproductive Medicine of Jean-Verdier University Hospital, France. All patients undergoing FET with HRT for endometrial preparation with Vaginal P only from November 9th, 2018 to March 19th, 2020 were included. Embryos obtained from oocyte donation or in vitro maturation oocytes were excluded.
Study design
We conducted a retrospective analysis of data collected during this period. Data were extracted from electronic patient files. Patient characteristics recorded were age, body mass index (BMI), HRT cycle parameters, biological data, and outcomes. The primary endpoint was the comparison of LBR between patients with normal P levels with “in phase” transfers and patients with low P levels supplemented by DYD with postponed transfers. Secondary outcomes were: positive pregnancy test, fetal heartbeat on ultrasound at 6-8 weeks (clinical pregnancy), ongoing pregnancy rate at 12 weeks, mean delivery term (weeks of gestation (WG)), and mean birth weight (g). This retrospective study received approval from an institutional review board on 2 July 2020.
Protocol of endometrial preparation
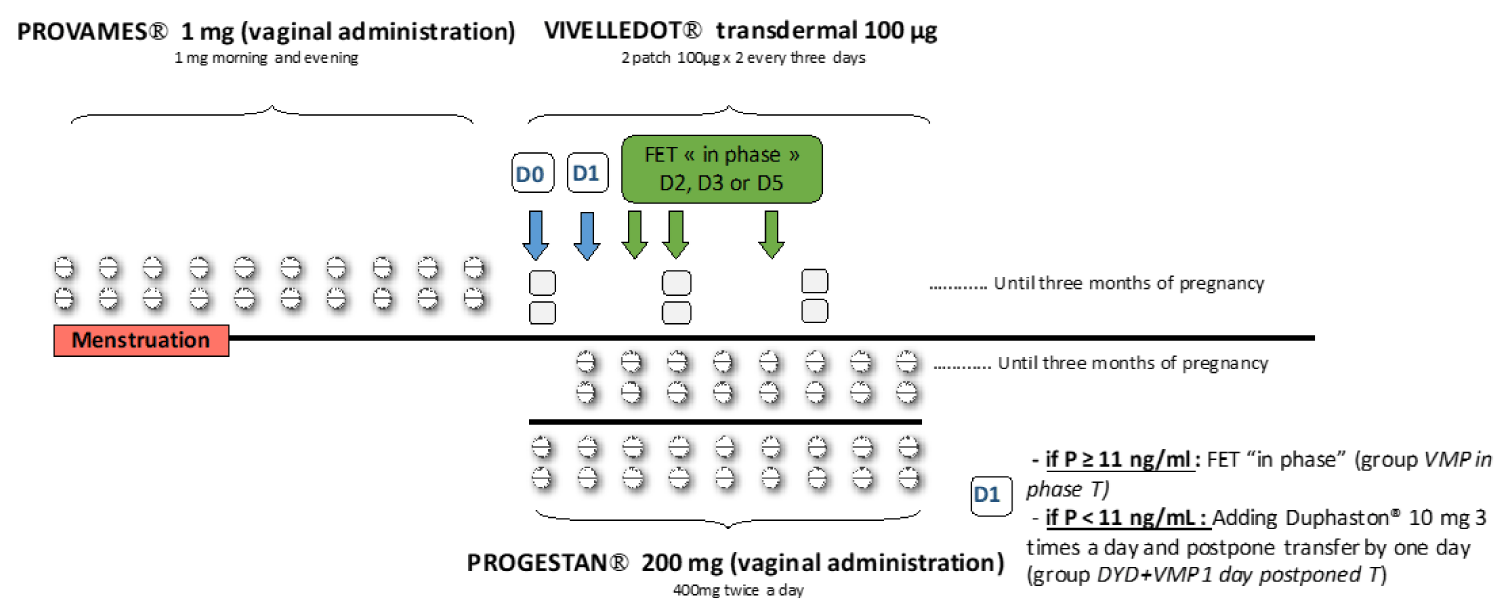
Vaginal estrogen administration (Provames® Merus Labs Luxco, Luxembourg, Luxembourg)1 mg twice a day was started on the first day of a natural menstrual cycle (without previous down-regulation with GnRH agonist). After 10 to 12 days of treatment, monitoring was performed by blood sample to measure estradiol (E2), P, and LH levels, and by vaginal ultrasound to assess endometrial thickness. If endometrial thickness was < 7 mm, estrogen supplementation was prolonged up to adequate endometrium or the cycle was canceled. If endometrial thickness was ≥ 7 mm with a triple-line pattern and serum P levels were lower than 1.5 ng/mL, vaginal micronized P (Progestan® Besins international, Montrouge, France) 400 mg twice a day was initiated in the evening (day-0 of P administration D0) and estrogen administration was switched from the vaginal to the transdermal route (Vivelledot® Novartis Pharma, Rueil-Malmaison, France) 100 µg patch x 2 every three days. Serum P was measured in the morning (between 7h 30 and 8h 30) on the day following exogenous P introduction (day-1 of P administration, D1) after two vaginal intakes, 1 at bedtime and 1 at sunrise.
Our previous receiver operating characteristic (ROC) curve of serum P levels on FET day showed that the optimal P level was 13 ng/mL to maximize sensitivity and specificity for live birth [19]. However, since P levels on D1 after P administration were on average 2 ng/mL lower than the following days (D2 of P administration or on FET day), we opted for a P threshold at 11 ng/mL. When P levels were ≥ 11 ng/mL, FET was performed on day 2 (D2) of progesterone administration for day-2 embryos, on day 3 (D3) for day-3 embryos, and on day 5 (D5) for blastocysts. When P levels were < 11 ng/mL, DYD (Duphaston® Mylan medical, Paris, France) 10 mg 3 times a day orally was added to vaginal P and FET was postponed by one day (Figure 1).
Figure 1: Endometrial preparation. Provames® is started on the first day of a natural menstrual cycle, after 10 days of treatment, a monitoring by blood sample to measure estradiol (E2), progesterone (P) and LH levels and by vaginal ultrasound is performed. If endometrial thickness was ≥ 7 mm with a triple-line pattern and serum P levels were lower than 1.5 ng/mL, Progestan® was initiated in the evening (day-0 of P administration D0) and estrogen administration was switched from the vaginal to the transdermal route (Vivelledot®). Serum P was measured in the morning on the day following exogenous P introduction after the second vaginal administration (day-1 of P administration, D1). When P levels were ≥ 11 ng/mL, FET was performed on day 2 (D2) of progesterone administration for day-2 embryos, on day 3 (D3) for day-3 embryos and on day 5 (D5) for blastocysts (group VMP in phase T. When P levels were < 11 ng/mL, Duphaston® was added to vaginal P and FET was postponed by one day (group DYD+VMP 1 day postponed T).
Serum hormonal measurement
Hormonal measurements were carried out using commercially available chemo-luminescence immunoassays with an automated Elecsys immune analyzer (ECLIA, Roche Diagnostics, Meylan, France). The sensitivity of the assay was 5 pg/ml for E2, 0.03 ng/ml for P, and 0.07 IU/l for LH. Intra- and inter-assay coefficients of variation were respectively 5% and 10% for E2, 3% and 5% for P and 2.3% and 2.6% for LH.
Embryo transfer
Embryos were issued from either in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) cycles. Embryos were thawed after vitrification on day-2, day-3, or at the blastocyst stage. All embryo transfers were guided by ultrasound. The number of transferred embryos, embryo stage, and embryo quality were recorded. Quality was considered Q+ if at least one embryo of good quality was transferred. Criteria for Q+ quality were the presence of 3 to 5 blastomeres cells without fragmentation (type A) for day-2 embryos, the presence of 6 to 10 blastomeres cells without fragmentation, or less than 20% fragmentation (type A and B) according to the Holte classification for day-3 embryos [40]. Criteria for Q+ quality for blastocyst were embryos classified B4-B5-B6 and AA, AB, BB (expanded to hatched blastocyst, and quality A or B for inner cell mass and trophectoderm) according to the Gardner classification [42].
Statistical analysis
Outcomes of FET were compared between patients with “in phase” transfers and patients who received additional DYD and whose transfer was postponed. Results from the descriptive analysis were expressed as mean ± SD in tables. Analyses were performed using Pearson chi-square tests for nominal variables and Student T-tests for continuous variables. A P - value < 0.05 was considered statistically significant. Analyses were performed with StatView (Abacus Concepts, Berkeley, CA, USA).
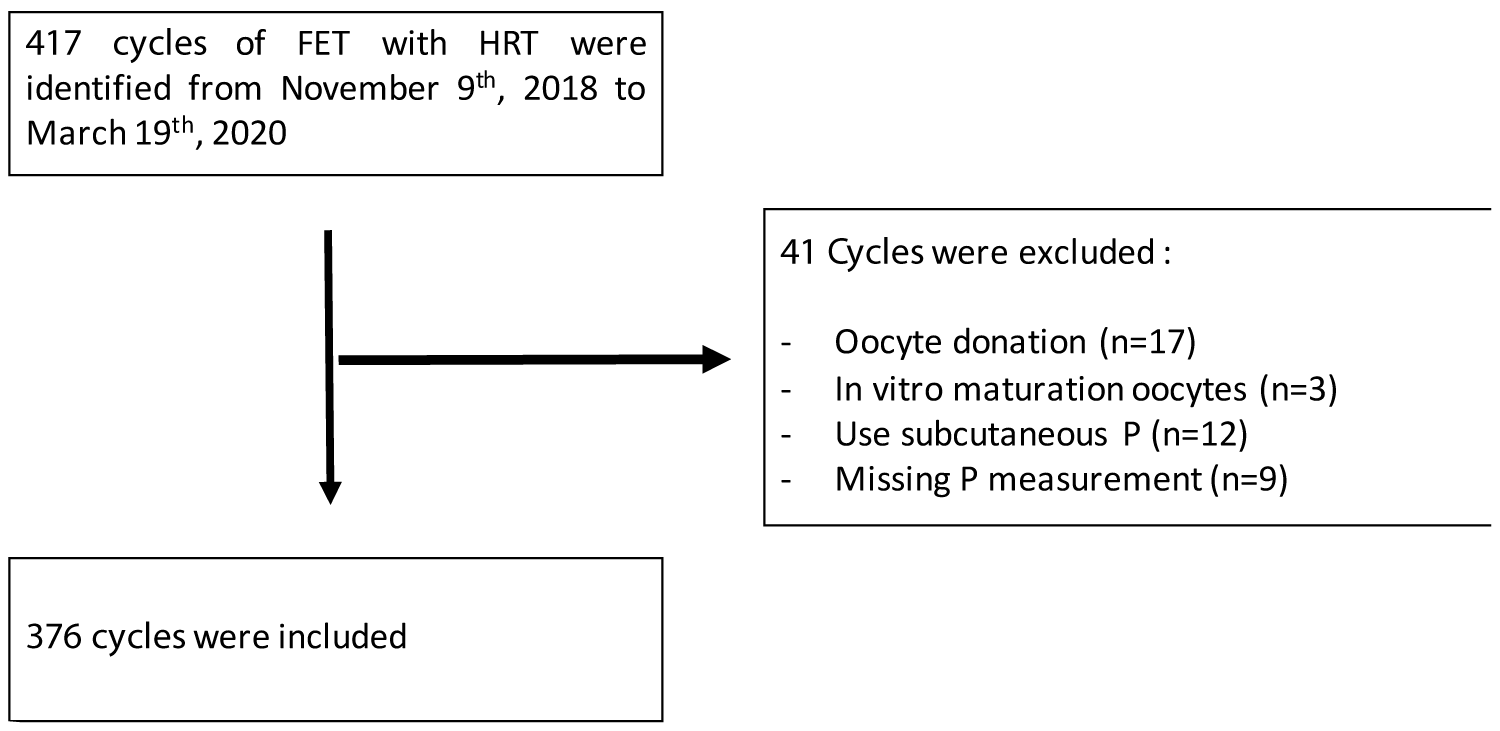
417 cycles of FET with HRT were performed from November 9th, 2018 to March 19th, 2020. Cycles were excluded in the case of oocyte donation (n = 17), in vitro maturation oocytes (n = 3), use of subcutaneous P (n = 12), or missing P measurement (n = 9). The final analysis included 376 cycles (314 patients) (Figure 2).
Figure 2: Flowchart. FET: Frozen Embryo Transfer; HRT: Hormonal Replacement Therapy; P: Progesterone.
DYD was added to vaginal micronized P (VMP) in 237 cycles (group VMP+DYD 1 day postponed T). The outcome of these 237 cycles were compared to the outcome of cycles with P > 11 ng/ml on D1 that received VMP only and subsequent “in phase” transfers (group VMP in phase T, 139 cycles) (Table 1).
| Table 1: Characteristics of patients, FET cycles according to «VMP+DYD 1 day postponed transfer» or « VMP in phase transfer ». | |||
| Characteristics | VMP In phase T (n = 139)Mean (+ SD) or N (%) | VMP+DYD 1 day postponed T (n = 237)Mean (+ SD) or N (%) | P |
| Patients Age (y) BMI (kg/m²) |
34.0 + 4.9 25.1 + 4.3 |
34.1 + 4.9 24.8 + 4.8 |
NS NS |
| Prior to P introduction E2 (pg/mL) P (ng/mL) Endometrium (mm) |
1431 + 730 0.2 + 0.2 9.7 + 2.1 |
1241 + 714 0.2 + 0.2 9.7 + 2.2 |
0.014 NS NS |
| D1 post P introduction P (ng/mL) E2 (pg/mL) |
13.9 + 2.9 392 + 355 |
8 + 1.9 287 + 226 |
0.0001 0.0005 |
| ET Embryo number (n) D2/ D3/blastocysts n (%) Good quality embryos |
1.5 + 0.6 7/45/87 (5/32.4/62.6) 95/139 (68.3 %) |
1.4 + 0.5 13/60/164 (5.5/25.3/69.2) 175/237 (73.8 %) |
0.008
NS NS |
| VMP: Vaginal Micronized Progesterone; DYD: Dydrogesterone; T: Transfer; FET: Frozen Embryo Transfer; NS: Non-Significant; E2: Oestradiol; P: Progesterone; D1: Day-1 Of Progesterone Administration; D2: Day-2 Of Progesterone Administration; D3: Day-3 Of Progesterone Administration. | |||
Characteristics of patients in both groups were similar for age, BMI, P levels, and endometrial thickness prior to P introduction, embryo transfer stage, and proportion of good quality embryos (Table 1). However, a slightly but significantly lower number of embryos were transferred in the group VMP+DYD 1 day postponed T (1.4 + 0.5 vs. 1.5 + 0.6, P = 0.008), in relation with a slightly lower number of blastocysts transferred (1.3 + 0.5 vs. 1.4 + 0.5, P = 0.013).
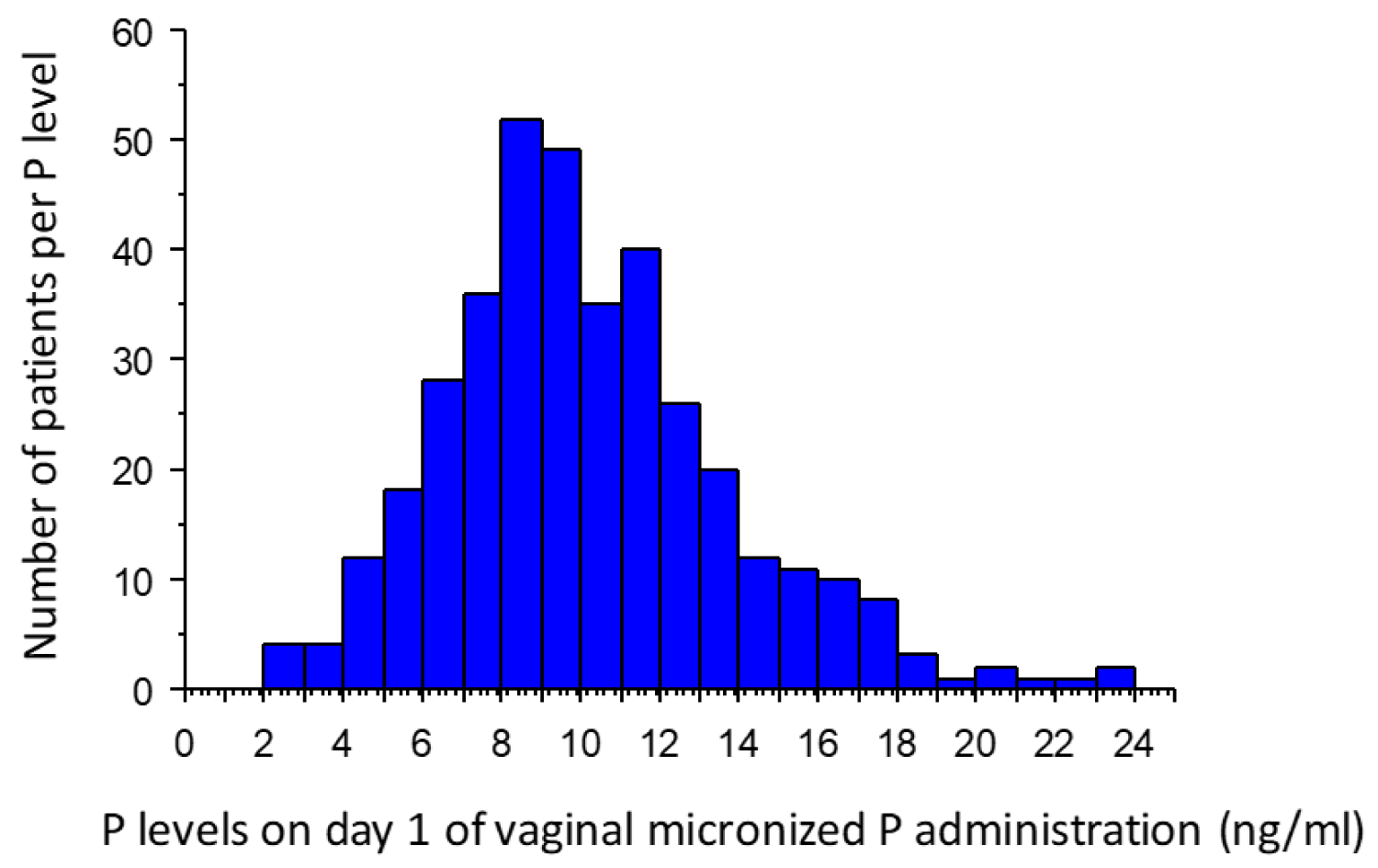
Mean serum P level on D1 was 10.2 + 3.7 ng/mL (range 2.6-25.3 ng/ml) (Figure 3). Mean P level in the group VMP+DYD 1 day postponed T was 8 + 1.9 ng/mL and in the group VMP in phase T 13.9 + 2.9 ng/mL (P < 0.0001). Serum E2 levels were also significantly lower in patients with P < 11 ng/mL (group VMP+DYD 1 day postponed T) both after vaginal administration (prior to P introduction) (1241 + 714 vs. 1431 + 730 pg/mL, P = 0.014) and after transdermal admini-stration (after introduction of P) (287 + 226 vs. 392 + 355 pg/mL, P = 0.005) (Table 1).
Figure 3: Histogram of P level on day 1 of vaginal micronized P administration (ng/mL) in the total population. P: Progesterone.
Regarding the primary endpoint, this protocol led to a similar LBR between the group VMP+DYD 1 day postponed and the group VMP in phase T (26.1 vs. 27.3%, NS) (Table 2).
Table 2: Outcomes according to « VMP+DYD addition and 1 day postponed transfer » or « VMPin phase T ». |
|||
| Outcomes | VMP In phase T (n = 139)N (%) | VMP+DYD 1 day postponed T (n = 237)N (%) | P |
Positive pregnancy test Ø Singleton (n) ØTwin (n) |
53/139 (38.1%) 34 4 |
105/237 (44.3%) 57 5 |
NS
NS
NS |
VMP: Vaginal Micronized Progesterone; DYD: Dydrogesterone; T: Transfer; NS: Non-Significant; LBR: Live Birth Rate; WG: Weeks of Gestation. |
|||
Concerning secondary endpoints, no difference between the group VMP+DYD 1 day postponed and the group VMP in phase T was found in terms of positive pregnancy tests (44.3 vs. 38.1%, NS), clinical pregnancy (29.1 vs. 28.8%, NS), or ongoing pregnancy rates at 12 weeks (27.0 vs. 28.1%, NS). While there was no significant difference in the term of delivery between the two groups, the birth weight of singletons was significantly higher in the group VMP+DYD 1 day postponed T (Table 2).
In order to ensure that dydrogesterone supplementation was effective in patients with P levels lower than the 25th percentile of P levels (7.7 ng/mL), outcomes among patients with P levels < 11 ng/mL were compared according to this threshold and they did not significantly differ (Table 3).
| Table 3: Outcomes between patients with P levels lower or higher than 25th percentile of P levels (7.7 ng/ml) in group VMP+DYD 1 day postponed. | |||
| HRT FET | P levels < 25th percentile (n = 91)N (%) | P levels > 25th percentile (n = 146)N (%) | p |
| Positive pregnancy test (%) Clinical pregnancy (%) Ongoing pregnancy (%) Live birth rate (%) |
38/91 (41.7) 27/91 (29.7) 26/91 (28.6) 26/91 (28.6) |
67/146 (45.9) 42/146 (28.8) 38/146 (26.0) 36/146 (24.7) |
NS NS NS NS |
| P: Progesterone; VMP: Vaginal Micronized Progesterone; DYD: Dydrogesterone | |||
The present study shows that the adjunction of oral P, DYD, associated with the postponement of FET by one day might optimize the outcome of patients with low P levels in HRT-FET cycles and avoid the cancellation of a large number of cycles in case of low P level. Indeed, despite a slightly lower number of transferred embryos, the outcome of FET was similar in patients with low P levels supplemented with DYD with subsequent embryo transfer postponed by 1 day compared to patients with normal P levels and “in phase” transfers. Hence, DYD seems to be efficient as a luteal P support in patients with low P levels following VMP although delaying embryo transfer could also play a role to overcome impaired outcomes associated with low P levels.
Previous studies have shown that low P levels following vaginal administration were associated with significantly decreased ongoing pregnancy or LBR [16–25]. Labarta, et al. [20] in a large prospective study showed that patients receiving micronized P need to reach a minimum of 8.8 ng/ml circulating P level to maintain pregnancy in HRT cycles. Considering the optimal P threshold determined by ROC or sensitivity analyses, values ranged from 9.8 to 15 ng/mL (9.8 ng/ml for Maignien, et al. [21]; for 10.4 ng/mL for Labarta, et al. [20], 10.64 ng/mL for Gaggiotti-Marre, et al. [18]; 11 ng/mL for Alsbjerg, et al. [23]; 13.5 ng/mL for Cédrin-Durnerin, et al. [19], 15 ng/mL for Yovich, et al. [16] and Basnayake, et al. [22]). In contrast, one recent study [43] concluded that P levels do not influence outcomes independent of a 10 ng/mL threshold, but P doses were adapted in patients with P levels < 8 ng/mL. About one-third (from 25% up to more than 50%) of patients receiving vaginal P experienced low P levels and impaired outcomes. In our study, 63% of patients with P < 11 ng/mL on day 1 were supplemented with DYD. This proportion is higher than percentages reported previously but similar to the proportion of patients (72%) below the cut-off of 13.5 ng/mL at FET time determined by ROC curve analysis in our previous study [19]. On the other hand, deleterious effect of high P values above a certain ceiling was unfrequently observed with vaginal P and only with high administered doses (pessaries at 1200 mg daily for Yovich, et al. [16] and gel 360 mg daily for Alsbjerg, et al. [28]).
In our study, we observed that patients with low P levels on day 1 of P administration have significantly lower E2 levels both after vaginal administration of E2 (prior to P introduction) and after transcutaneous administration of E2 (after P introduction), suggesting a lower absorption of steroid hormones through cutaneous and mucosal epitheliums. A recent retrospective cohort study showed that weight, age, time of blood sampling and a history of low progesterone are factors associated with progesterone levels before frozen embryo transfer of blastocysts [26]. Moreover, there was no significant difference in BMI between the two groups. Conversely, a retrospective study of 707 single euploid frozen-warmed blastocyst transfers reported decreased LBR in case of high BMI [44].
Since, vaginal P absorption is limited [14], adding P via another route of administration could be useful to overcome the negative impact of low P levels. Indeed, Labarta, et al. [29] first demonstrated that adjunction of subcutaneous P on the day of embryo transfer was effective in patients with low P levels to normalize outcomes. In a recent prospective cohort [30] and a case-control study [31], individualized luteal phase support through the addition of subcutaneous P in cases of low serum P values the day prior to FET similarly resulted in excellent LBR similarly to women with adequate P levels. It was also previously reported [45] that adding intramuscular to vaginal P administration appeared to increase the LBR in oocyte donation. Results of a recent prospective randomized study in FET confirmed these data [46]. On the other hand, systematic combined administration of SC [47] or IM [9] P to vaginal P optimized serum P levels and weakened the effects of serum P levels on LBR. However, with systematic administration of high doses of vaginal and IMP [48], a lower LBR was reported in patients with P > 32.5 ng/mL suggesting that P levels have to stay in a window of P levels. Furthermore, combined P treatments have the disadvantage of both the burden of daily injections and the cost of treatment for patients.
DYD is an oral retroprogesterone, a selective P receptor agonist, simple, well-tolerated, and safe. This treatment does not need further control of P level since DYD does not interfere with serum P measurement. Indeed, due to structural differences between DYD and P, DYD cannot be quantified by tests used in routine to determine progesterone levels [49]). DYD is used in a variety of indications worldwide such as recurrent miscarriages, luteal insufficiency, endometriosis, or menstrual disorders. It is estimated that 113 million women and about 20 million fetuses have been exposed to DYD since 1960 [37]. Compared with oral micronized P, DYD has a better oral bioavailability [33] and a greater affinity for P receptors. Therefore, lower oral doses are required to promote endometrial proliferation [50]. DYD also has a lower affinity for androgen and glucocorticoid receptors [32], showing a favorable safety and tolerability profile during pregnancy. Data from prospective studies for luteal phase support in IVF show that DYD is well tolerated overall and obtains a higher patient satisfaction compared to micronized vaginal P [51,52]. Recently, results of Phase III clinical studies comparing DYD (30 mg) to micronized vaginal P capsules (600 mg) (Lotus I [37]) and DYD (30 mg) to micronized vaginal P gel (90 mg) (Lotus II [36]) for luteal support in fresh IVF cycles demonstrated the non-inferiority of oral DYD. Furthermore, no safety concerns related to DYD used during early pregnancy were reported. These Phase III studies showed that the incidence of serious adverse events and congenital, familial, and genetic disorders were similar between DYD and micronized vaginal P capsule or gel groups for luteal phase support. Nevertheless, a retrospective case-control study investigating the use of DYD in early pregnancy to prevent miscarriages reported a positive association between congenital heart malformation and DYD [53]. However, some biases have to be considered in the interpretation of these results. Indeed, miscarriages are an important risk factor for congenital heart defects and the presence of exposure to DYD was recorded based on the mother’s declarations. As such, no causal relationship can be affirmed. In FET cycles, a recent prospective cohort study [54] showed that systematic addition of DYD (20 mg daily) to VMP led to higher live birth rates and lower miscarriage rates compared to VMP alone. It seems therefore that routine prescription of the association VPM+DYD could be an alternative to serum P measurement and individualized supplementation. Finally, DYD alone could be used to supplement artificial cycles but limited data are available in artificial cycles with only two studies led on relatively small effect. A randomized study [39] reported comparable pregnancy rates at the dose of 40 mg/day between DYD and micronized vaginal progesterone at the dose of 800 mg/day. Conversely, results of another small randomized study [40] comparing the use of 20 mg/day of DYD and micronized vaginal group at the dose of 800 mg/day were disappointing. Therefore, further studies are required to investigate the efficiency of luteal phase support by DYD only in artificial cycles. The lack of difference in outcomes of patients supplemented with DYD with P levels lower or higher than 7.7 ng/mL seems reassuring on the efficacy of the dose of 30 mg/day administered in our study. Nevertheless, the development of a specific assay to measure serum DYD levels would be useful to assess potential variations of bio-disponibility between patients.
Despite our study design modifying both P support and timing of embryo transfer, its retrospective nature, and the sample size, these results suggest that serum P measurement prior to ET followed by further addition of DYD to vaginal P and postponement of transfer might optimize the outcome of patients with low P levels in hormonally substituted FET cycles. Further studies are required to assess the optimal timing of P measurement and optimal threshold of P level according to the nature of P supplementation in order to personalize luteal phase replacement in hormonally substituted cycles. A systematically combined route of administration could also be a suitable alternative to personalized luteal support.
- Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017; 23: 139-155. PubMed: https://pubmed.ncbi.nlm.nih.gov/27827818/
- Belva F, Bonduelle M, Roelants M, Verheyen G, Van Landuyt L. Neonatal health including congenital malformation risk of 1072 children born after vitrified embryo transfer. Hum Reprod. 2016; 31: 1610–1620. PubMed: https://pubmed.ncbi.nlm.nih.gov/27165622/
- Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update. 2013; 19: 433–457. PubMed: https://pubmed.ncbi.nlm.nih.gov/23827986/
- Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Gynaecology and Fertility Group, editor. Cochrane Database Syst Rev. 2017; 7: CD003414. PubMed: https://pubmed.ncbi.nlm.nih.gov/28675921/
- Groenewoud ER, Cohlen BJ, Macklon NS. Programming the endometrium for deferred transfer of cryopreserved embryos: hormone replacement versus modified natural cycles. Fertil Steril. 2018; 109: 768–774. PubMed: https://pubmed.ncbi.nlm.nih.gov/29778369/
- Yarali H, Polat M, Mumusoglu S, Yarali I, Bozdag G. Preparation of endometrium for frozen embryo replacement cycles: a systematic review and meta-analysis. J Assist Reprod Genet. 2016; 33: 1287–1304. PubMed: https://pubmed.ncbi.nlm.nih.gov/27549760/
- Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, et al. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Gynaecology and Fertility Group, editor. Cochrane Database Syst Rev. 2020; 10: CD006359. PubMed: https://pubmed.ncbi.nlm.nih.gov/20091592/
- Kahraman S, Karagozoglu SH, Karlıkaya G. The efficiency of progesterone vaginal gel versus intramuscular progesterone for luteal phase supplementation in gonadotropin-releasing hormone antagonist cycles: a prospective clinical trial. Fertil Steril. 2010; 94: 761–763. PubMed: https://pubmed.ncbi.nlm.nih.gov/19939363/
- Polat M, Mumusoglu S, Bozdag G, Ozbek IY, Humaidan P, et al. Addition of intramuscular progesterone to vaginal progesterone in hormone replacement therapy in vitrified–warmed blastocyst transfer cycles. Reprod Biomed Online. 2020; 40: 812–818. PubMed: https://pubmed.ncbi.nlm.nih.gov/32362573/
- Shapiro DB, Pappadakis JA, Ellsworth NM, Hait HI, Nagy ZP. Progesterone replacement with vaginal gel versus i.m. injection: cycle and pregnancy outcomes in IVF patients receiving vitrified blastocysts. Hum Reprod. 2014; 29: 1706–1711. PubMed: https://pubmed.ncbi.nlm.nih.gov/24847018/
- Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Crinone vaginal gel is equally effective and better tolerated than intramuscular progesterone for luteal phase support in in vitro fertilization–embryo transfer cycles: a prospective randomized study. Fertil Steril. 2010; 94: 2596–2599. PubMed: https://pubmed.ncbi.nlm.nih.gov/20347079/
- Miles RA, Paulson RJ, Lobo RA, Press MF, Dahmoush L, et al. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: a comparative study**Presented at the 40th Annual Meeting of the Pacific Coast Fertility Society, Indian Wells, California, April 8 to 12, 1992. Fertil Steril. 1994; 62: 485–490. PubMed: https://pubmed.ncbi.nlm.nih.gov/8062942/
- Nahoul K, Dehennin L, Jondet M, Roger M. Profiles of plasma estrogens, progesterone and their metabolites after oral or vaginal administration of estradiol or progesterone. Maturitas. 1993; 16: 185–202. PubMed: https://pubmed.ncbi.nlm.nih.gov/8515718/
- Paulson RJ, Collins MG, Yankov VI. Progesterone Pharmacokinetics and Pharmacodynamics With 3 Dosages and 2 Regimens of an Effervescent Micronized Progesterone Vaginal Insert. J Clin Endocrinol Metab. 2014; 99: 4241–4249. PubMed: https://pubmed.ncbi.nlm.nih.gov/24606090/
- Duijkers IJM, Klingmann I, Prinz R, Wargenau M, Hrafnsdottir S, et al. Effect on endometrial histology and pharmacokinetics of different dose regimens of progesterone vaginal pessaries, in comparison with progesterone vaginal gel and placebo. Hum Reprod. 2018; 33: 2131-2140. PubMed: https://pubmed.ncbi.nlm.nih.gov/30265306/
- Yovich JL, Conceicao JL, Stanger JD, Hinchliffe PM, Keane KN. Mid-luteal serum progesterone concentrations govern implantation rates for cryopreserved embryo transfers conducted under hormone replacement. Reprod Biomed Online. 2015; 31: 180–191. PubMed: https://pubmed.ncbi.nlm.nih.gov/26099447/
- Labarta E, Mariani G, Holtmann N, Celada P, Remohí J, et al. Low serum progesterone on the day of embryo transfer is associated with a diminished ongoing pregnancy rate in oocyte donation cycles after artificial endometrial preparation: a prospective study. Hum Reprod. 2017; 32: 2437–2442. PubMed: https://pubmed.ncbi.nlm.nih.gov/29040638/
- Gaggiotti-Marre S, Martinez F, Coll L, Garcia S, Álvarez M, et al. Low serum progesterone the day prior to frozen embryo transfer of euploid embryos is associated with significant reduction in live birth rates. Gynecol Endocrinol. 2019; 35: 439–442. PubMed: https://pubmed.ncbi.nlm.nih.gov/30585507/
- Cédrin-Durnerin I, Isnard T, Mahdjoub S, Sonigo C, Seroka A, et al. Serum progesterone concentration and live birth rate in frozen–thawed embryo transfers with hormonally prepared endometrium. Reprod Biomed Online. 2019; 38: 472–480. PubMed: https://pubmed.ncbi.nlm.nih.gov/30642638/
- Labarta E, Mariani G, Paolelli S, Rodriguez-Varela C, Vidal C, et al. Impact of low serum progesterone levels on the day of embryo transfer on pregnancy outcome: a prospective cohort study in artificial cycles with vaginal progesterone. Hum Reprod. 2021; 36: 683–692. PubMed: https://pubmed.ncbi.nlm.nih.gov/33340402/
- Maignien C, Bourdon M, Marcellin L, Laguillier-Morizot C, Borderie D, et al. Low serum progesterone affects live birth rate in cryopreserved blastocyst transfer cycles using hormone replacement therapy. Reprod Biomed Online. 2021; S1472-6483(21)00579-4. PubMed: https://pubmed.ncbi.nlm.nih.gov/34980570/
- Basnayake SK, Volovsky M, Rombauts L, Osianlis T, Vollenhoven B, et al. Progesterone concentrations and dosage with frozen embryo transfers - What’s best? Aust N Z J Obstet Gynaecol. 2018; 58: 533–538. PubMed: https://pubmed.ncbi.nlm.nih.gov/29271471/
- Alsbjerg B, Thomsen L, Elbaek HO, Laursen R, Povlsen BB, et al. Progesterone levels on pregnancy test day after hormone replacement therapy-cryopreserved embryo transfer cycles and related reproductive outcomes. Reprod Biomed Online. 2018; 37: 641–647. PubMed: https://pubmed.ncbi.nlm.nih.gov/30385142/
- Commissaire M, Epelboin S, Vigan M, Tubiana S, Llabador MA, et al. Serum progesterone level and ongoing pregnancy rate following frozen-thawed embryo transfer after artificial endometrial preparation: a monocentric retrospective study. J Gynecol Obstet Hum Reprod. 2020; 49: 101828. PubMed: https://pubmed.ncbi.nlm.nih.gov/32534215/
- Labarta E, Rodriguez-Varela C, Mariani G, Bosch E. Serum progesterone profile across the mid and late luteal phase in artificial cycles is associated with pregnancy outcome. Front. Endocrinol. 2021; 12: 665717. PubMed: https://pubmed.ncbi.nlm.nih.gov/34177806/
- González-Foruria I, Gaggiotti-Marre S, Álvarez M, Martínez F, García S, et al. Factors associated with serum progesterone concentrations the day before cryopreserved embryo transfer in artificial cycles. Reprod Biomed Online. 2020; 40: 797–804. PubMed: https://pubmed.ncbi.nlm.nih.gov/32386938/
- Labrosse J, Peigné M, Eustache F, Sifer C, Grynberg M, et al. Women utilizing oocyte donation have a decreased live birth rate if they displayed a low progesterone level in a previous hormonal replacement mock cycle. J Assist Reprod Genet. 2021; 38: 605–612. PubMed: https://pubmed.ncbi.nlm.nih.gov/33415529/
- Alsbjerg B, Thomsen L, Elbaek HO, Laursen R, Povlsen BB, et al. Can combining vaginal and rectal progesterone achieve the optimum progesterone range required for implantation in the HRT-FET model? Reprod Biomed Online. 2020; 40: 805–811. PubMed: https://pubmed.ncbi.nlm.nih.gov/32376312/
- Labarta E, Mariani G, Rodríguez-Varela C, Bosch E. Individualized luteal phase support normalizes live birth rate in women with low progesterone levels on the day of embryo transfer in artificial endometrial preparation cycles. Fertil Steril. 2022; 117: 96-103. PubMed: https://pubmed.ncbi.nlm.nih.gov/34548167/
- Álvarez M, Gaggiotti-Marre S, Martínez F, Coll L, García S, et al. Individualised luteal phase support in artificially prepared frozen embryo transfer cycles based on serum progesterone levels: a prospective cohort study. Hum Reprod. 2021; 36: 1552-1560. PubMed: https://pubmed.ncbi.nlm.nih.gov/33686413/
- Yarali H, Polat M, Mumusoglu S, Ozbek IY, Erden M, et al. Subcutaneous luteal phase progesterone rescue rectifies ongoing pregnancy rates in hormone replacement therapy vitrified–warmed blastocyst transfer cycles. Reprod Biomed Online. 2021; 43: 45-51. PubMed: https://pubmed.ncbi.nlm.nih.gov/34016521/
- Rižner TL, Brožič P, Doucette C, Turek-Etienne T, Müller-Vieira U, et al. Selectivity and potency of the retroprogesterone dydrogesterone in vitro. Steroids. 2011; 76: 607–615. PubMed: https://pubmed.ncbi.nlm.nih.gov/21376746/
- Stanczyk FZ, Hapgood JP, Winer S, Mishell DR. Progestogens Used in Postmenopausal Hormone Therapy: Differences in Their Pharmacological Properties, Intracellular Actions, and Clinical Effects. Endocr Rev. 2013; 34: 171–208. PubMed: https://pubmed.ncbi.nlm.nih.gov/23238854/
- Barbosa MWP, Silva LR, Navarro PA, Ferriani RA, Nastri CO, et al. Dydrogesterone vs progesterone for luteal-phase support: systematic review and meta-analysis of randomized controlled trials: Dydrogesterone for assisted reproduction. Ultrasound Obstet Gynecol. 2016; 48: 161–170. PubMed: https://pubmed.ncbi.nlm.nih.gov/26577241/
- Griesinger G, Tournaye H, Macklon N, Petraglia F, Arck P, et al. Dydrogesterone: pharmacological profile and mechanism of action as luteal phase support in assisted reproduction. Reprod Biomed Online. 2019; 38: 249–259. PubMed: https://pubmed.ncbi.nlm.nih.gov/30595525/
- Griesinger G, Blockeel C, T. Sukhikh G, Patki A, Dhorepatil B, et al. Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial. Hum Reprod. 2018; 33: 2212-2221. PubMed: https://pubmed.ncbi.nlm.nih.gov/30304457/
- Tournaye H, Sukhikh GT, Kahler E, Griesinger G. A Phase III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum Reprod. 2017; 32: 1019–1027. PubMed: https://pubmed.ncbi.nlm.nih.gov/28333318/
- van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Gynaecology and Fertility Group, editor. Cochrane Database Syst Rev. 2015: CD009154. PubMed: https://pubmed.ncbi.nlm.nih.gov/26148507/
- Rashidi BH, Ghazizadeh M, Tehrani Nejad ES, Bagheri M, Gorginzadeh M. Oral dydrogesterone for luteal support in frozen-thawed embryo transfer artificial cycles: A pilot randomized controlled trial. Asian Pac J Reprod. 2016; 5: 490–494.
- Zarei A, Sohail P, Parsanezhad ME, Alborzi S, Samsami A, et al. Comparison of four protocols for luteal phase support in frozen-thawed Embryo transfer cycles: a randomized clinical trial. Arch Gynecol Obstet. 2017; 295: 239–246. PubMed: https://pubmed.ncbi.nlm.nih.gov/27761732/
- Holte J, Berglund L, Milton K, Garello C, Gennarelli G, et al. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum Reprod. 2007; 22: 548–557. PubMed: https://pubmed.ncbi.nlm.nih.gov/17095516/
- Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts: Curr Opin Obstet Gynaecol. 1999; 11: 307–311. PubMed: https://pubmed.ncbi.nlm.nih.gov/10369209/
- Volovsky M, Pakes C, Rozen G, Polyakov A. Do serum progesterone levels on day of embryo transfer influence pregnancy outcomes in artificial frozen-thaw cycles? J Assist Reprod Genet. 2020; 37: 1129–1135. PubMed: https://pubmed.ncbi.nlm.nih.gov/32043182/
- Boynukalin FK, Gultomruk M, Cavkaytar S, Turgut E, Findikli N, et al. Parameters impacting the live birth rate per transfer after frozen single euploid blastocyst transfer. Viganò P, editor. PLOS ONE. 2020; 15: e0227619. PubMed: https://pubmed.ncbi.nlm.nih.gov/31929583/
- Delcour C, Robin G, Delesalle AS, Drumez E, Plouvier P, et al. Weekly intramuscular progesterone for luteal phase support in women receiving oocyte donation is associated with a decreased miscarriage rate. Reprod Biomed Online. 2019; 39: 446–451. PubMed: https://pubmed.ncbi.nlm.nih.gov/31311693/
- Devine K, Richter KS, Jahandideh S, Widra EA, McKeeby JL. Intramuscular progesterone optimizes live birth from programmed frozen embryo transfer: a randomized clinical trial. Fertil Steril. 2021; 116: 633-643. PubMed: https://pubmed.ncbi.nlm.nih.gov/33992421/
- Ramos NN, Pirtea P, Benammar A, Ziegler D de, Jolly E, et al. Is there a link between plasma progesterone 1–2 days before frozen embryo transfers (FET) and ART outcomes in frozen blastocyst transfers? Gynecol Endocrinol. 2020; 1–4. PubMed: https://pubmed.ncbi.nlm.nih.gov/32996332/
- Alyasin A, Agha-Hosseini M, Kabirinasab M, Saeidi H, Nashtaei MS. Serum progesterone levels greater than 32.5 ng/mL on the day of embryo transfer are associated with lower live birth rate after artificial endometrial preparation: a prospective study. Reprod Biol Endocrinol. 2021; 19: 24. PubMed: https://pubmed.ncbi.nlm.nih.gov/33602270/
- Abdel-Hamid ME, Sharaf LH, Kombian SB, Diejomaoh FME. Determination of Dydrogesterone in Human Plasma by Tandem Mass Spectrometry: Application to Therapeutic Drug Monitoring of Dydrogesterone in Gynecological Disorders. Chromatographia. 2006; 64: 287–292. PubMed: https://pubag.nal.usda.gov/catalog/4392298
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, et al. Classification and pharmacology of progestins. Maturitas. 2003; 46: 7–16. PubMed: https://pubmed.ncbi.nlm.nih.gov/14670641/
- Chakravarty BN, Shirazee HH, Dam P, Goswami SK, Chatterjee R, et al. Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: Results of a randomised study. J Steroid Biochem Mol Biol. 2005; 97: 416–420. PubMed: https://pubmed.ncbi.nlm.nih.gov/16213136/
- Tomic V, Tomic J, Klaic DZ, Kasum M, Kuna K. Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2015; 186: 49–53. PubMed: https://pubmed.ncbi.nlm.nih.gov/25622239/
- Zaqout M, Aslem E, Abuqamar M, Abughazza O, Panzer J, et al. The Impact of Oral Intake of Dydrogesterone on Fetal Heart Development During Early Pregnancy. Pediatr Cardiol. 2015; 36: 1483–1488. PubMed: https://pubmed.ncbi.nlm.nih.gov/25972284/
- Vuong LN, Pham TD, Le KTQ, Ly TT, Le HL, et al. Micronized progesterone plus dydrogesterone versus micronized progesterone alone for luteal phase support in frozen-thawed cycles (MIDRONE): a prospective cohort study. Hum Reprod. 2021; 36: 1821–1831. PubMed: https://pubmed.ncbi.nlm.nih.gov/33930124/