More Information
Submitted: February 07, 2022 | Approved: March 08, 2022 | Published: March 09, 2022
How to cite this article: Gomes RR. Journey with a 21 weeks primi with acute massive pulmonary thromboembolism secondary to possible “Latent Lupus”: an audacious ride. Clin J Obstet Gynecol. 2022; 5: 022-026.
DOI: 10.29328/journal.cjog.1001102
Copyright License: © 2022 Gomes RR. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pregnancy; Pulmonary embolism; Thrombolysis; Streptokinase; Latent lupus
Journey with a 21 weeks primi with acute massive pulmonary thromboembolism secondary to possible “Latent Lupus”: an audacious ride
Richmond R Gomes*
Associate Professor, Medicine, Ad-Din Women's Medical College Hospital, Dhaka, Bangladesh
*Address for Correspondence: Dr. Richmond Ronald Gomes, Associate Professor, Medicine, Ad-Din Women's Medical College Hospital, Dhaka, Bangladesh, Email: [email protected]
In pregnancy, the incidence of pulmonary embolism (PE) is increased fivefold when compared to nonpregnant women of the same age, and PE is one of the leading causes of death during pregnancy.
However, the diagnosis of PE among pregnant women is complicated by concerns regarding radiation exposure. Systemic lupus erythematosus (SLE) is an autoimmune disorder with a wide array of presentations and a predilection to affect women of certain ethnic backgrounds. The hallmark of the disease is multisystem involvement, dispersed in time and severity. Usual pulmonary involvement includes pleuritis, pleural effusions, pneumonitis, shrinking lung syndrome, pulmonary hypertension, and alveolar hemorrhage. Pulmonary embolism (PE) is a relatively unusual presentation of SLE. We report the case of a 20-year-old primi at 21 weeks gestation with an acute PE with central chest pain and shortness of breath. The absence of overt signs and symptoms and traditional risk factors prompted a fragmentary workup. This led to the detection of antibodies sensitive for SLE, in the absence of overt signs and symptoms. We revive the concept of latent lupus, a condition construed as early lupus. We firmly suspect direct causation between SLE and PE. Further studies are needed to establish pathogenesis to facilitate early diagnosis and prevent morbidity and mortality from PE. Due to persistent hypotension, thrombolytic therapy with streptokinase was administered and the clinical and hemodynamic response was excellent, with no maternal or fetal hemorrhagic complications. The clinical presentation of pulmonary embolism is sometimes camouflaged by the physiological changes that occur in pregnancy and diagnosis is often delayed by a reluctance to expose the fetus to ionizing radiation.
Venothromboembolic diseases remain one of the leading causes of maternal mortality. It is estimated that 0.2% - 4% of pregnancies in the Western world are complicated by cardiovascular disease, and this figure is increasing. There is frequently a need for diagnostic and/or therapeutic cardiological intervention in pregnant women, and this is always a challenge, both because most cardiologists lack experience with this patient group and because few cardiological interventions have been thoroughly validated in this population. Pulmonary embolism (PE) is the third most common cause of cardiovascular mortality. Traditional risk factors include immobilization, active malignancy, trauma, congestive heart failure, and others, which account for 74% of cases of venous thromboembolism (VTE), leaving 25% of cases unexplained [1]. Systemic lupus erythematosus (SLE) is an autoimmune disorder with a variety of manifestations. SLE usually presents with fatigue, arthralgia, arthritis, myalgia, and weight loss, but outside the musculoskeletal system, the pulmonary system is the next most commonly affected [2]. SLE and other autoimmune diseases have been associated with increased risk of VTE, and this report shows a likely association between SLE and PE.
A 20-year-old Bangladeshi overweight female, primi at 21 weeks of gestation with no comorbidities, no relevant family history and not taking any regular medication presented with sudden-onset, sharp retrosternal chest pain, and shortness of breath for one day. She denied any cough, fever, chills, hemoptysis, calf swelling, or leg pain. She reported no family history of bleeding, clotting, or rheumatologic disorders, no drug allergies, and toxic habits. She had no history of joint pain, oral ulcer, skin rash, or previous abortion. She was never on oral contraceptive medications but she had a history of traveling by bus to her hometown for almost 11 hours 2 days before presentation. On physical examination, she was agitated, hypotensive (70/40 mmHg), tachycardia (150 bpm), hypoxemia (oxygen saturation in room air 84%), and tachypneic (50 CPM). Jugular venous distension was seen. No signs of deep vein thrombosis (DVT) were observed. Her body mass index (BMI) was 29. On examination, her breath sounds were decreased bilaterally due to pain. Her neurological examination was normal and no skin rashes, joint swelling, or oral ulcers were noted. Precordium examination revealed no abnormalities. Laboratory tests are listed in Table 1.
| Table 1: Laboratory investigations. | |
| Investigations | Results |
| Complete blood count | Hemoglobin 10.6 g/dl Platelet 190 × 103/l ESR- 29 mm in 1st hour |
| D-dimer | 6.61 mg/l (normal < 0.5 mg/l) |
| Arterial blood gas(on room air) | pH 7.49, pO2 69 mmHg; pCO2 29 mmHg, HCO3 – 17.5 mmol/l |
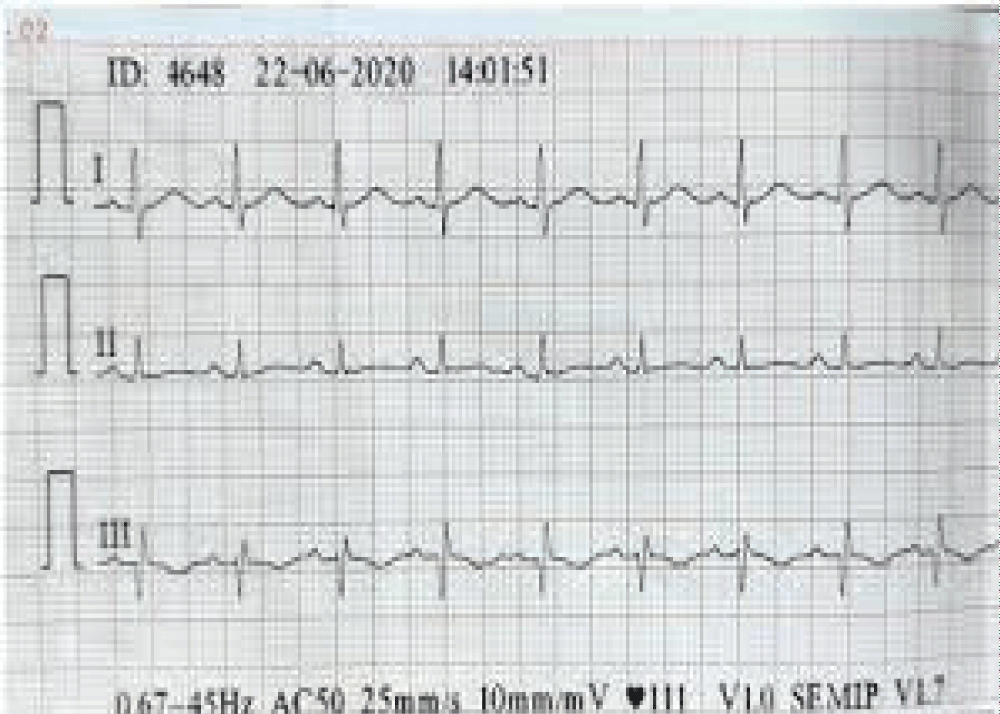
| ECG | inverted T waves and Q waves in lead III and S wave in lead with sinus tachycardia (Figure 1) |
| Doppler venous ultrasonography of lower limbs | Normal |
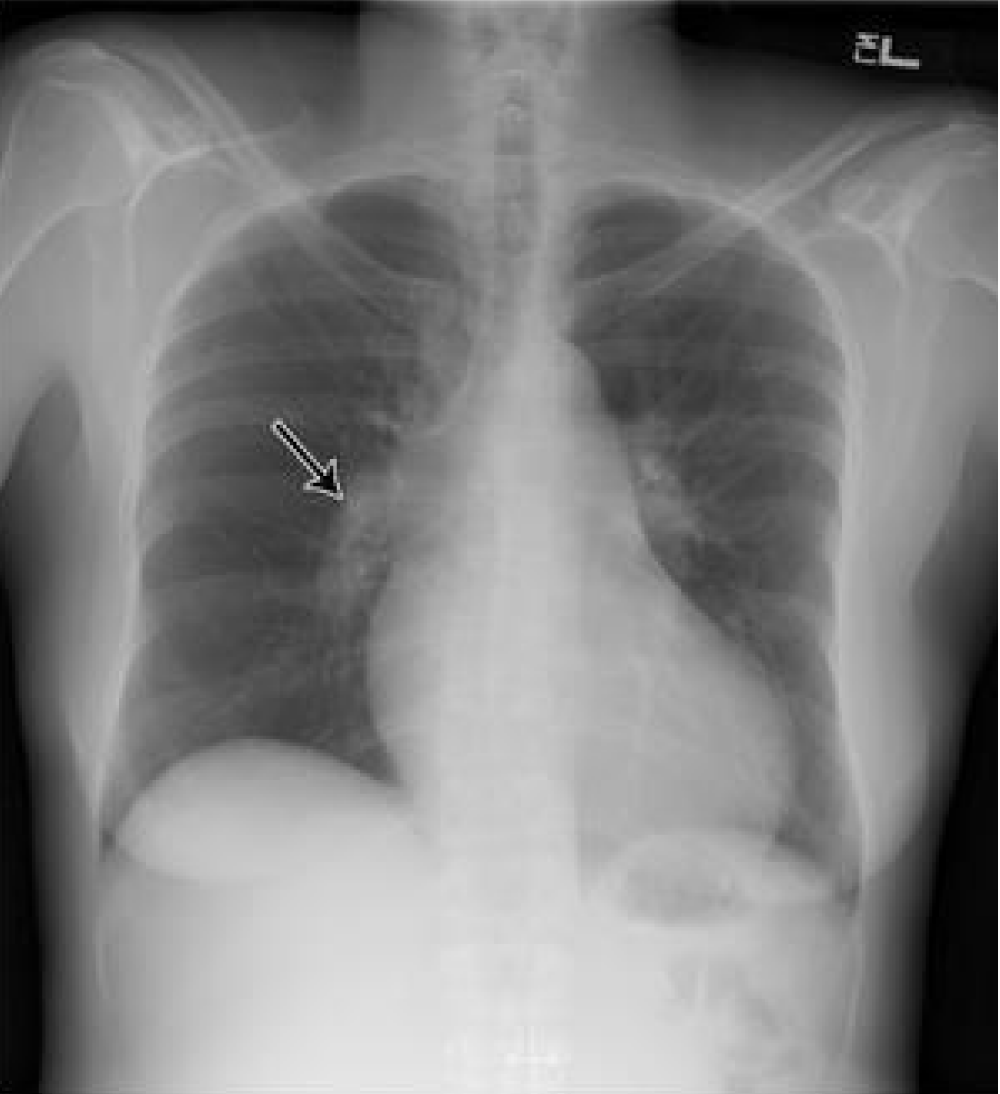
| Chest X-ray | Enlarged main pulmonary artery (Figure 2) |
| pro-brain natriuretic peptide | 127 pg/mL |
| ANA | moderately positive (neocleoli pattern) |
| anti-dS DNA, lupus anticoagulant, anticardiolipin antibody, protein C, antithrombin 3, C3, C4, ENA (extractable nuclear antigen) | Negative |
| Urine routine examination | No proteinuria, no casts, no RBC’s |
| Serum creatinine | 0.83 mg/dl |
| Fibrinogen | 510 mg/L(normal upto 500 mg/L) |
| protein S | 40% (normal 50% - 120%) |
| transthoracic echocardiography(TTE) | Moderate to large pulmonary embolism in main pulmonary artery 8 mm distal to the pulmonary valve with mildly enlarged RA and RV(RV 36 mm). |
Figure 1: Electrocardiography showing sinus tachycardia, inverted T waves, and Q waves in lead III and S wave in lead I.
Figure 2: Chest X-ray P/A view showing Fleischner’s sign(white arrow- enlarged main pulmonary artery) Bedside transthoracic echocardiography(TTE) showed moderate to large pulmonary embolism in the main pulmonary artery 8 mm distal to the pulmonary valve with mildly enlarged RA and RV(RV 36 mm).
The fetus was in a transverse position, with good vital signs; the cardiotocograph was reactive, with good variability and no uterine contraction. The ultrasound scan showed concordant fetal growth. CTPA(CT pulmonary angiogram was not done due to financial constraints. According to the Wells score, the patient’s clinical probability of acute pulmonary thromboembolism (PTE) was intermediate (score 6) (Table 2).
| Table 2: The Wells scoring system for diagnosis of PTE. | |
| Clinical signs of DVT | 3.0 |
| No alternate diagnosis is likely or more likely than PTE | 3.0 |
| Heart rate > 100 bpm | 1.5 |
| Immobilization in the previous 3 days or surgery in the previous 4 weeks | 1.5 |
| Previous diagnosis of DVT/PTE | 1.5 |
| Hemoptysis | 1.0 |
| Cancer | 1.0 |
| Clinical probability: 0-1, low; 2-6, intermediate; > 7, high. DVT: Deep Vein Thrombosis; PTE: Pulmonary Thromboembolism. | |
After weighing the hemorrhagic risk against the greater risk of irreversible clinical decompensation, it was decided to administer thrombolytic therapy with streptokinase(patient could not afford tenecteplase) 1.5 million units over 1 hour with bolus isotonic fluid support. Within three hours clear clinical and hemodynamic improvement was seen, with blood pressure 95/65 mmHg, heart rate 100 bpm, breathing rate 22 breaths/min, and decreasing need for supplementary oxygen therapy. Later she was started subcutaneous enoxaparin 1 mg/kg, which was later bridged to rivaroxaban. She remained hemodynamically stable throughout her hospital stay, with 98% oxygen saturation in room air. No maternal or fetal hemorrhagic complications occurred. On the fifth day, ECG and TTE were performed, which showed a mildly enlarged RV and no signs of pulmonary hypertension or pulmonary embolus. She was discharged on the 10th day. There is a plan to continue rivaroxaban up to 6 weeks postpartum and follow her up regularly on an outpatient door basis for the future development of overt SLE.
Bedside transthoracic echocardiography(TTE) showed moderate to large pulmonary embolism in the main pulmonary artery 8 mm distal to the pulmonary valve with mildly enlarged RA and RV(RV 36 mm).
Pregnancy is known to increase the risk for thromboembolism by 4-10 times [3-5] compared to the non-pregnant state. This risk is present from the first trimester [6] and is at the highest in the third trimester [4]. Postpartum, this risk increases 100-fold in the immediate first-week post partum [3] and remains at 20- to 80-fold until 6 weeks post partum [3,4]. From the sixth week, the risk is the same as for non-pregnant women [7].
VTE(venous thromboembolism), which includes DVT(deep vein thrombosis) and PTE(pulmonary thromboembolism), is the leading cause of maternal death (20%) in developed countries, accounting for 1.2 - 4.7 deaths per 100000 pregnancies [6]. The precise incidence of VTE is unknown but is estimated at 0.5-2 cases per 1000 pregnancies [7].
The most feared manifestation of venous thromboembolism (VTE) is PTE, a common entity with a mortality of 30% if untreated, mainly due to recurrence. Anticoagulation at therapeutic doses within 24 hours reduces mortality to 2% - 8% [8,9]. In-hospital mortality is 5% - 17% in patients who present evidence of RV dysfunction at diagnosis [10] and 20% - 30% in those with hemodynamic compromise [11]. The presenting symptoms include dyspnea (73%), pleuritic chest pain (47%), cough (43%), calf or thigh pain (up to 42%), calf or thigh swelling (up to 39%), and wheezing (31%) [12].
There are three pathophysiological mechanisms, known as Virchow’s triad, that together or in isolation may be responsible for the high incidence of VTE in pregnancy [13]:
(1) Venous stasis: This begins in the first trimester and reaches a maximum at 36 weeks. It is caused by progesterone-induced venodilation, compression of the pelvis by the gravid uterus, and pulsatile compression by any of the iliac arteries on the left iliac vein (which explains why 80% of cases of DVT in pregnancy are on the left, a phenomenon known as May-Thurner syndrome) [14].
(2) Vascular injury: During childbirth, the veins of the pelvic region may be distended and/or traumatized, especially when a cesarean section is performed (which explains the greater risk described above).
(3) Hypercoagulability: The production of several coagulation factors (I, II, VII, VIII, IX, and X) increases in pregnancy, while protein S production and the activity of the inhibitors of fibrinolysis PAI-1 and PAI-2 are reduced. These physiological changes are crucial to the hemodynamic challenges of birth (peripartum bleeding is the leading cause of maternal death in developing countries) [15]. This prothrombotic state will be further exacerbated by the presence of hereditary thrombophilia such as factor V Leiden, the G20210A mutation in the prothrombin gene, antithrombin III, or protein C or S deficiency, or the presence of antiphospholipid antibodies [13]. Level of protein S falls and level of fibrinogen level rises physiologically during the second trimester of pregnancy which may contribute to PTE in our patient.
SLE is a chronic inflammatory autoimmune disorder that affects multiple organ systems. Pulmonary involvement usually develops late in the disease course and can affect any part of the pulmonary system, including the air airways, lung parenchyma, pulmonary vasculature, pleura, and diaphragm [17]. Patients with SLE are at increased risk of venous thromboembolism, with a prevalence of 9% [18], and patients with aPL(antiphospholipid antibody) have an even higher increased risk of 35% to 42% [19]. aPL may be present in up to two-thirds of patients with lupus [20]. Due to heterogeneity, accurate diagnosis of SLE has always been a clinical challenge. The latest criteria for SLE comes from the European League Against Rheumatism (EULAR)/American College of Rheumatology classification, which has been reported to have a sensitivity of 96.1% and specificity of 93.4% [21].
Our patient had none of the usual signs and symptoms of SLE. An autoimmune workup was requested out of academic inquisitiveness, which was positive for high titer ANA but negative for anti dS DNA. ENA profile, complements, aPL all were negative. Hence, the EULAR criteria for SLE diagnosis did not apply to our patient.
We want to revisit the concept of “latent lupus” at this juncture, which is believed to be an evolutionary phase of SLE, with a high risk of development of SLE in a few years [22]. Routine monitoring of patients who do not meet full criteria currently can aid in early diagnosis and management [23]. The overall risk of pulmonary embolism in the first year after admission for an autoimmune disorder is reported to be 6.38 (95% CI 6.19-6.57). The risk was particularly high with SLE at 10.23 (95% CI 8.31-12.45). There is a suggestion that these autoimmune conditions should be managed as hypercoagulable states. Also, the risk of VTE seems to reduce over the years since diagnosis, further emphasizing the importance of a timely diagnosis [24]. There are currently no established predictors of VTE in SLE, except for a pilot study that followed levels of galectin-3-binding protein (G3BP). G3BP is believed to induce a prothrombotic state by activating type I interferon. The reported hazard ratio for increased risk of VTE was 1.18 (95% CI: 1.05-1.33, p = 0.007) [25].
The clinical features of VTE can be frustratingly difficult to evaluate since most healthy pregnant women have lower limb edema and up to 70% suffer from shortness of breath during pregnancy [26]. Diagnosis of VTE, and particularly PTE, requires a high index of clinical suspicion, based on predisposing conditions and risk factors (in the case presented, these included overweight, pregnancy at age over 35, thrombophilia, immobility during a flight, and initial symptoms in the left leg compatible with DVT) [8,27]. Based on risk factors and physical examination, the clinical probability of PTE can be calculated using the Wells or Geneva score. This then guides the choice of diagnostic exams (Table 1) [8]. These tools have not been validated in the pregnant patient, although one study has found three variables that appear to predict DVT in pregnant women: left leg symptoms, > 2 cm difference in thigh circumference, and first trimester [28]. Laboratory results such as respiratory alkalosis or elevated fibrin degradation products are also commonly found in healthy pregnant women; levels of the latter increase with gestational age and reach a maximum at the time of birth, but such tests should be performed due to their ability to exclude disease and to avoid unnecessary exposure to ionizing radiation [27,29].
Diagnosis
The deep venous system of the lower limbs is difficult to assess by physical examination, and when DVT and/or PTE are suspected the techniques used are B-mode echocardiography and compression venous ultrasonography together with color Doppler in transverse view [8,27]. Magnetic resonance imaging has 100% sensitivity in diagnosing DVT and appears to be safe in pregnancy [30]. Documented DVT in a hemodynamically stable pregnant woman is sufficient motive to begin OAC(oral anticoagulation) without needing to exclude or confirm PTE, although at least 70% of patients with PTE do not have DVT at the time of diagnosis [31].
Exams using ionizing radiation in women of childbearing age should be performed during the first 10 days after a menstrual cycle, and if there is a possibility that the woman is pregnant, this must be excluded first. Exposure of the ovaries to radiation pre-conception has no measurable effects on future gestations, and the risk from ionizing radiation to pregnant women is the same as to those who are not pregnant. However, for the fetus, ionizing radiation can cause death, malformations (particular ocular), growth retardation, and mutagenic and carcinogenic effects, which depend mainly on gestational age (the most vulnerable period is between the second and eighth week) and the absorbed radiation dose. A major problem with the diagnosis of PTE is clinicians’ reluctance to expose the fetus to ionizing radiation, often due to overestimation of the risk of harm. When faced with the clinical probability of PTE, the primary diagnostic modalities are pulmonary ventilation-perfusion scintigraphy (VPS) and thoracic CT. The estimated radiation dose from CT absorbed by the fetus is 0.003-0.13 mGy, while from VPS it is 0.2 mGy. There is no evidence that doses of up to 50 mGy lead to fetal abnormalities, low IQ, growth restriction, or miscarriage. Less radiation is absorbed by the mother’s mammary and pulmonary tissue with VPS than with CT [32,33]. Although VPS and CT appear to be safe for the fetus, it should be noted that some studies suggest that exposure to low radiation doses in utero can increase the risk of childhood leukemia (1 in 2000 compared to the baseline risk of 1 in 2800), which does not compare with the risk of maternal death from undiagnosed and untreated PTE (15%) [34]. In a pregnant woman with a normal chest X-ray, VPS may be more valuable in diagnosing PTE than CT, since in the latter exam the contrast material can be interrupted by unspecified blood from the inferior vena cava. Conversely, CT should be used when the chest X-ray is abnormal since it can diagnose other conditions such as pneumonia or another lung disease. Pulmonary angiography should not be used in pregnancy [27,35]. Iodinated contrast agents may lead to fetal thyroid dysfunction (although this has never been reported with isolated use), and this should be assessed in the first week after birth.
Pulmonary thromboembolism is common in pregnancy and is associated with significant maternal morbidity and mortality. It should always be considered in the presence of suspicious symptoms and signs and confirmed by appropriate diagnostic investigations. The diagnosis of PE among pregnant women is complicated by concerns regarding radiation exposure. Through this report, we aim to emphasize that acute pulmonary embolism could be the initial presentation of systemic lupus erythematosus and other autoimmune disorders. Physicians should keep a low threshold to suspect SLE and other autoimmune disorders, even in the absence of overt clinical features, in patients with unexplained VTE. Oral anticoagulation should be begun immediately, and thrombolysis should be considered in cases of hemodynamic instability as it has been shown to be effective in the few cases described in the literature and the case presented here.
- Heit JA, O'Fallon WM, Petterson TM, Lohse CM, Silverstein MD, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism. A population-based study. 3rd. Arch Intern Med. 2002; 162: 1245–1248. PubMed: https://pubmed.ncbi.nlm.nih.gov/12038942/
- Metry AM, Al Salmi I, Al Balushi F, Yousef MA, Al Ismaili F, et al. Systemic lupus erythematosus: symptoms and signs at initial presentations. Antiinflamm Antiallergy Agents Med Chem. 2019; 18: 142–150. PubMed: https://pubmed.ncbi.nlm.nih.gov/30488801/
- Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Inter Med. 2005; 143: 697-706. PubMed: https://pubmed.ncbi.nlm.nih.gov/16287790/
- Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJM. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thrombo Haemosta. 2008; 6: 632-637. PubMed: https://pubmed.ncbi.nlm.nih.gov/18248600/
- Martinelli I, De Stefano V, Taioli E, Paciaroni K, Rossi E, et al. Inherited thrombophilia and first venous thromboembolism during pregnancy and puerperium. Thrombo Haemosta. 2002; 87: 791-795. PubMed: https://pubmed.ncbi.nlm.nih.gov/12038778/
- James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstetr Gynecol. 2006; 194: 1311-1315. PubMed: https://pubmed.ncbi.nlm.nih.gov/16647915/
- Jacobsen AF, Skjeldestad FE, Sandset PM. Ante- and postnatal risk factors of venous thrombosis: a hospital-based case-control study. J Thromb Haemost. 2008; 6: 905-912. PubMed: https://pubmed.ncbi.nlm.nih.gov/18363820/
- The Task Force for the Diagnosis, Management of Acute Pulmonary Embolism of the European Society of Cardiology. Guidelines on diagnosis and management of acute pulmonary embolism. Eur Heart J. 2008; 29: 2276-2315. PubMed: https://pubmed.ncbi.nlm.nih.gov/18757870/
- Laack TA, Goyal DG. Pulmonary embolism: an unsuspected killer. Emerg Med Clin N Am. 2004; 22: 961-983. PubMed: https://pubmed.ncbi.nlm.nih.gov/15474778/
- Kasper W, Konstantinides S, Geibel A, Tiede N, Krause T, et al. Prognostic significance of right ventricular afterload stress detected by echocardiography in patients with clinically suspected pulmona embolism. Heart. 1997; 77: 346-469. PubMed: https://pubmed.ncbi.nlm.nih.gov/9155614/
- Fedullo PF, Tapson VF. The evaluation of suspected pulmonary embolism. New Engl J Med. 2003; 349: 1247-1256. PubMed: https://pubmed.ncbi.nlm.nih.gov/14507950/
- Stein PD, Beemath A, Matta F, Weg JG, Yusen RD, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II Am J Med. 2007; 120: 871–879. PubMed: https://pubmed.ncbi.nlm.nih.gov/17904458/
- Aird WC. Vascular bed-specific thrombosis. J Thromb Haemost. 2007; 5: 283-291. PubMed: https://pubmed.ncbi.nlm.nih.gov/17635738/
- Macklon NS, Greer IA, Bowman AW. An ultrasound study of gestational and postural changes in the deep venous system of the leg in pregnancy. Br J Obstet Gynaecol. 1997; 104: 191-197. PubMed: https://pubmed.ncbi.nlm.nih.gov/9070137/
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006; 367: 1066-1074. PubMed: https://pubmed.ncbi.nlm.nih.gov/16581405/
- Walker MC, Garner PR, Keely EJ. Thrombosis in pregnancy: a review. J Soc Obstet Gynaecol Can. 1998; 20: 943-952.
- Gari AG, Telmesani A, Alwithenani R. Pulmonary manifestations of systemic lupus erythematosus. In: Almoallim H, ed. Systemic lupus erythematosus. Rijeka, Croatia. InTech. 2012; 313-336.
- Gladman DD, Urowitz MB. Venous syndromes and pulmonary embolism in systemic lupus erythematosus. Ann Rheum Dis. 1980; 39: 340-343. PubMed: https://pubmed.ncbi.nlm.nih.gov/7436559/
- Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med. 1990; 112: 682-698. PubMed: https://pubmed.ncbi.nlm.nih.gov/2110431/
- Ruiz-Irastorza G, Egurbide MV, Ugalde J, Aguirre C. High impact of antiphospholipid syndrome on irreversible organ damage and survival of patients with systemic lupus erythematosus. Arch Intern Med. 2004; 164: 77-82. PubMed: https://pubmed.ncbi.nlm.nih.gov/14718326/
- Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019; 78: 1151–1159. PubMed: https://pubmed.ncbi.nlm.nih.gov/31383717/
- Ganczarczyk L, Urowitz MB, Gladman DD. Latent lupus. J Rheumatol. 1989; 4: 475–478. PubMed: https://pubmed.ncbi.nlm.nih.gov/2746587/
- Bertsias GK, Pamfil C, Fanouriakis A, Boumpas DT. Diagnostic criteria for systemic lupus erythematosus: has the time come? Nat Rev Rheumatol. 2013; 9: 687–694. PubMed: https://pubmed.ncbi.nlm.nih.gov/23838616/
- Zöller B, Li X, Sundquist J, Sundquist K. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 2012; 9812: 244–249. PubMed: https://pubmed.ncbi.nlm.nih.gov/22119579/
- Peretz ASR, Rasmussen NS, Jacobsen S, Sjöwall C, Nielsen CT. Galectin-3-binding protein is a novel predictor of venous thromboembolism in systemic lupus erythematosus. Clin Exp Rheumatol. 2021; 39: 1360-1368. PubMed: https://pubmed.ncbi.nlm.nih.gov/33337998/
- Weinberger SE, Weiss ST, Cohen WR, Weiss JW, Johnson TS. Pregnancy and the lung. Am Rev Respir Dis. 1980; 121: 559-581. PubMed: https://pubmed.ncbi.nlm.nih.gov/6998334/
- European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM); Regitz-Zagrosek V, Lundqvist CB, et al. ESC guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011; 32: 3147-3197. PubMed: https://pubmed.ncbi.nlm.nih.gov/21873418/
- Chan WS, Lee A, Spencer FA, Crowther M, Rodger M, et al. Predicting deep venous thrombosis in pregnancy: out in ‘‘LEFt’’ field? Ann Intern Med. 2009; 151: 85-92. PubMed: https://pubmed.ncbi.nlm.nih.gov/19620161/
- Kline JA, Williams GW, Hernandez-Nino J. D-dimer concentrations in normal pregnancy: new diagnostic thresholds are needed. Clin Chem. 2005; 51: 825-829. PubMed: https://pubmed.ncbi.nlm.nih.gov/15764641/
- Spritzer CE, Evans AC, Kay HH. Magnetic resonance imaging of deep venous thrombosis in pregnant women with lower extremity edema. Obstet Gynecol. 1995; 85: 603-607. PubMed: https://pubmed.ncbi.nlm.nih.gov/7898841/
- Turkstra F, Kuijer PM, van Beek EJ, Brandjes DP, ten Cate JW, et al. Diagnostic utility of ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann Intern Med. 1997; 126: 775-781. PubMed: https://pubmed.ncbi.nlm.nih.gov/9148650/
- ACOG, Committee on Obstetric Practice. Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol. 2004; 104: 647-651. PubMed: https://pubmed.ncbi.nlm.nih.gov/15339791/
- Ratnapalan S, Bona N, Chandra K, Koren G. Physicians’ perceptions of teratogenic risk associated with radiography and CT during early pregnancy. AJR Am J Roentgenol. 2004; 182: 1107-1109. PubMed: https://pubmed.ncbi.nlm.nih.gov/15100102/
- Harvey EB, Boice Jr JD, Honeyman M, Flannery JT. Prenatal X-ray exposure and childhood cancer in twins. N Engl J Med. 1985; 312: 541-545. PubMed: https://pubmed.ncbi.nlm.nih.gov/3969117/
- U-King-Im JM, Freeman SJ, Boylan T, Cheow HK. Quality of CT pulmonary angiography for suspected pulmonary embolus in pregnancy. Eur Radiol. 2008; 18: 2709-2715. PubMed: https://pubmed.ncbi.nlm.nih.gov/18651151/