More Information
Submitted: March 17, 2021 | Approved: April 07, 2021 | Published: April 09, 2021
How to cite this article: Jangra I, Chauhan A, Prabha V. Synergistic interactions of sperm impairing bacteria: Impact on pregnancy outcome in mouse model. Clin J Obstet Gynecol. 2021; 4: 033-039.
DOI: 10.29328/journal.cjog.1001083
Copyright License: © 2021 Jangra I, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Synergistic interactions of sperm impairing bacteria: Impact on pregnancy outcome in mouse model
Isheeta Jangra, Aditi Chauhan and Vijay Prabha*
Department of Microbiology, Panjab University, Chandigarh-160014, India
*Address for Correspondence: Dr. Vijay Prabha, Professor, Department of Microbiology, Panjab University, Chandigarh -160014. India, Tel: 91-9417065675; Email: [email protected]; [email protected]
Earlier in our laboratory, the role of various individual sperm impairing microorganisms on sperm parameters and female infertility has been elucidated at higher doses. As, multiple bacterial species tend to exert more pathogenic effect in comparison to single organism hence, present study was carried out to evaluate that if consortia of these sperm impairing organisms can lead to infertility in female mice at sub fertility dose. For this, impact of individual bacterial strains of Escherichia coli, Pseudomonas aeruginosa, Pseudomonas aeruginosa, Klebsiella pneumoniae and consortia of Escherichia coli and Pseudomonas aeruginosa, Escherichia coli and Pseudomonas aeruginosa, Escherichia coli and Klebsiella pneumoniae was examined on the motility, viability of mouse spermatozoa and fertility outcome. The results showed that the individual bacterial strains of E. coli, S. marcescens and K. pneumoniae could led to immobilization of spermatozoa by agglutination and P. aeruginosa led to immobilization of spermatozoa without agglutination. Also, all of them led to 100% sperm death in 45 min of incubation. In case of consortia of bacterial strains, the results showed sperm agglutination in all the cases and they were able to induce 100% sperm death at 30 min of incubation time. Further, in vivo studies were carried out to evaluate the impact of individual bacterial strains and consortia of bacterial strains on the fertility outcome in female Balb/c mice. For this, female mice were administered intravaginally with 101 cfu/20µl of individual bacterial strains or consortia of strains for 10 consecutive days or PBS. The results showed that both individual bacterial strains and consortia of bacterial strains were able to efficiently colonize the mouse vagina. Further, control group receiving phosphate buffer saline and groups receiving individual bacterial strains showed all the pregnancy related changes viz. abdominal distension, string of pearls on palpation as well as delivery of pups on completion of gestation period and delivery of pups. The histological examination of reproductive organs viz. uterus and ovary, of the female mice receiving PBS or individual bacterial strains showed the formation of corpus luteum in the ovary and the formation of decidua’s in the uterus, indicative of pregnancy. However, mice receiving consortia of bacterial strains did not show any pregnancy related changes throughout the experiment. Thus, these results indicate that the presence of consortia of sperm impairing microorganisms in vaginal milieu is efficient in provoking infertility even at subfertility doses.
The infectious diseases studied in humans and animals by the veterinarians, dentists, physicians and researchers have revealed that multiple pathogenic players are responsible for certain diseases. In the nineteenth century, the role of individual species of microbes in infectious diseases was documented by Robert Koch and Louis Pasteur. But over the past few decades, part played by the intricate communities of bacterial species in causing several complicated polymicrobial infections has been well established [1]. Polymicrobial infections involve polymicrobial communities in which two or more microorganisms act synergistically, or in succession, to mediate complex disease processes. These consortia of microbes typically coexist as combinations of communities of bacteria, viruses, protozoans and fungi in such a manner that their coexistence is facilitated by specific inter microbial and host interactions [2,3]. The microbes in these interactions exhibit ferocious competition for nutrients or cooperative mechanisms that support their mutual growth. The severe virulence traits, altered infected niche and assortment of host responses are stimulated, as a result of synergistic interactions among two or more etiologic agents in polymicrobial diseases [4]. Due to the far-reaching exploitation of culture dependent isolation techniques, most of the diseases were previously regarded as being monomicrobial in nature. Nevertheless, several are becoming renowned as true polymicrobial infections including diseases of the oral cavity, otitis media, diabetic foot wound infections, chronic infection in the cystic fibrosis lung and bacterial vaginosis with the instigation of culture-independent community analysis methodologies. Since the ecosystem of human vagina is highly complex composed of stratified squamous nonkeratinized epithelium and mixed aerobic and anaerobic bacteria which can affect the health of many generations and moreover it is exposed to external environment, many diverse bacterial communities harbour the vagina. Therefore, the incidence of these polymicrobial infections may be correlated with infertility also. Earlier in our laboratory, impact of individual uropathogens at higher doses on pregnancy outcome after intravaginal inoculation has been checked and the results showed that intravaginal inoculation with these microorganisms led to complete loss of fertility. This intrigued us to investigate the synergistic effects of consortia of various bacterial strains at lower doses on fertility outcome of female mice.
Microorganisms
The standard strains viz. Pseudomonas aeruginosa (MTCC 7641), Pseudomonas aeruginosa (MTCC 5342), Klebsiella pneumoniae (MTCC 4030) and a clinical isolate of Escherichia coli (isolated in our laboratory from semen of males undergoing analysis at PGIMER, Chandigarh) were used in the present study. The standard strains were obtained from Microbial Type Culture Collection, Institute of Microbial Technology, Sector 39, Chandigarh, India.
Animals and ethics
Sexually mature, 4-5 weeks old female weighing 22 ± 2g and 5-6 weeks old male weighing 25 ± 2 g Balb/c mice obtained from Central Animal House, Panjab University were used in the present study. The animals were kept under standard laboratory conditions with a photoperiod of 12h of light & 12 h of darkness and were housed in polypropylene cages, 6 mice per cage, with clean rice husk bedding, in the animal house. Only after the approval of the protocol by the Institutional Animals Ethics Committee of the Panjab University Chandigarh (IAEC/504 dated 02.04.14), experiments were performed in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Extraction of mouse spermatozoa
The 6–7-week-old BALB/c male mice were sacrificed by cervical dislocation, and spermatozoa from the vas deferens were collected in prewarmed 50 mM PBS by gentle teasing and the count was set at 40 × 106 spermatozoa/ml, unless stated otherwise.
Preparation of inoculum
All the bacterial strains viz. E. coli (E), S. marcescens (S), P. aeruginosa (P), K. pneumoniae (K) were grown in nutrient broth (10ml) at 37°C under shaking conditions. Cell pellet was collected by centrifugation at 10,000 rpm for 10 min and the pellets so obtained were washed twice with Phosphate Buffer Saline (PBS) (50 mM, pH 7.2) and resuspended in same buffer. Serial dilutions of washed cell pellets were made in PBS to adjust the final cfu for inoculum at 101 cfu per 20 µl of PBS.
Influence of individual bacterial strains and consortia of various bacterial strains on motility and viability
For this, equal volume of mouse spermatozoa (40 x 106 /ml) and bacterial strains (101 cfu of individual or consortia of bacterial strains) were mixed and the reaction mixture was incubated at 37 °C and examined after 5, 15 and 30 min. At each time interval, a wet preparation made with 10 µl of the mixture was observed for motility under a bright field microscope at 400X (Olympus). A control consisting of sterile PBS mixed with semen sample was set up simultaneously. Minimum ten fields were scanned and mean number of spermatozoa per field along with motility (%) were calculated. Motility of sperm was determined by the method of Emmens, [5].
A semi quantitative grading from no immobilization (-) to complete immobilization in which all motile sperms are immobilized (++++), was used.
Immobilization was graded, depending on the percentage of immotile spermatozoa observed in the microscopic fields, as follows:
Agglutination
Agglutination of spermatozoa occurs when the motile spermatozoa adhere to each other either head to head or tail to tail or in a mixed way i.e. head to tail. When immotile spermatozoa stick to each other or motile spermatozoa stick to mucus threads, cells other than spermatozoa, or debris, adherence is considered to be non-specific aggregation rather than agglutination and was recorded as such. Agglutination was assessed at the time of determining sperm motility. The type of agglutination was recorded e.g. head to head, tail to tail or mixed, and the agglutination was graded according to the intensity of the clumping observed in the microscopic field.
In order to assess the percentage of viable sperms, an equal volume of sperm sample was mixed with bacterial strains (individual and consortia of bacterial strains) and 0.5% eosin and examined for different time intervals (5, 15, 30, 45, 60 min) under the light microscope at 400X magnification. Any degree of pink/red stained spermatozoa was counted as ‘dead’ while unstained (white) as ‘alive’.
Investigation of the impact of individual bacterial strains and consortia of various bacterial strains on fertility outcome
Intravaginal inoculation: Female Balb/c mice were divided into eight groups (Groups I–VIII) comprising 3 mice in each group. Group III, III, IV, V of female mice served as control were intravaginally inoculated with PBS, 101 cfu/20µl of E. coli, 101 cfu/20 µl of S. marcescens, 101 cfu/20µl of P. aeruginosa and 101 cfu/20µl of K. pneumoniae, respectively, for 10 consecutive days. Group VI, VII, VIII were inoculated with consortia of bacterial strains Escherichia coli and Pseudomonas aeruginosa, Escherichia coli and Pseudomonas aeruginosa, Escherichia coli and Klebsiella pneumoniae, respectively for 10 consecutive days. To study the colonization pattern of these bacterial strains, vaginal lavages were taken from the female mice on every 3rd day viz. day 3, 6 and 9 which showed that the inoculated bacterial strains could efficiently persist in the mouse vagina till day 9. The isolates so obtained from the lavages were confirmed by reisolating on selective media. Control group I administered with PBS were culture negative throughout the experiment.
After 10 consecutive doses, bedding of male mice soiled with faeces and urine was put into the cages of female mice, so as to synchronise them into their oestrous cycle on day 11. On day 12, female mice were allowed to mate with proven male mice in a ratio of 2:1. Onset of pregnancy was considered by the presence of copulatory plug that give the confirmation of mating and this day was referred to as day 0 of gestation (GD 0).
The females in which the vaginal plug was observed were allowed to complete their gestation period (21 days) and they were examined for weight change, abdominal distension, string of pearls and delivery of pups over the period of gestation.
Histological examination of reproductive organs to assess pregnancy related changes
One mouse from each group was euthanized on day 14 of gestation, the reproductive organs (ovary and uterus) were collected and histological analysis was carried out. According to standard histological methods, the organs were fixed in 10% formaldehyde for 24 h and then embedded in paraffin. Serial paraffin sections were made, stained with haematoxylin/eosin and observed at X400 and X1000 magnification.
Influence of individual bacterial strains and consortia of various bacterial strains on motility and viability
Motility: Based on sperm microorganism interaction, microorganisms were observed to cause sperm immobilization via agglutination or without agglutination. Group I (PBS) did not show any negative influence on sperm motility at any of the specified time intervals. Group II (E. coli) showed immobilization via agglutination and the results revealed that coincubation of mouse spermatozoa with culture of E. coli could agglutinate 10%, 30% and 40% of spermatozoa after 5, 15 and 30 min of incubation, respectively. Group III (P. aeruginosa) showed immobilization without agglutination and the results revealed that 20% of sperm immobilization could be observed in 5 min, 40% of sperm immobilization in 15 min and 50% sperm immobilization in 30 min. Group IV (S. marcescens) demonstrated immobilization of spermatozoa via agglutination. The results showed 20% sperm agglutination after 5 min, 30% sperm agglutination after 15 min and 50% sperm agglutination could be observed after 30 min of incubation. Group V (K. pneumoniae) also led to immobilization of spermatozoa by agglutination. Here, 10% of sperm agglutination could be observed in 5 min, 25% in 15 min and 35% sperm agglutination in 30 min of incubation time.
The sperm agglutination in Group VI (E. coli + P. aeruginosa) was found to be 30% after 5, 15 and 30 min of incubation time as P. aeruginosa causes sperm immobilization without agglutination strain. The results showed that in Group VII (E. coli + S. marcescens), there was 40% of sperm agglutination in 5 min, 60% of sperm agglutination in 15 min and 70% sperm agglutination in 30 min. In case of Group VIII (E. coli + K. pneumoniae), the sperm agglutination was found to be 40%, 55% and 60% after 5, 15 and 30 min, respectively.
Viability: When the effect of these individual bacterial strains and consortia of bacterial strains on sperm viability was evaluated using eosin as the marker dye, the sperms appeared as either pink stained or unstained. In comparison to control (PBS), upon incubation of spermatozoa with individual bacterial strains (E. coli/S. marcescens/P. aeruginosa/K. pneumoniae), it was observed that all these bacterial strains were capable of inducing 100% sperm death in 45 min. On the other hand, when the effect of incubation of spermatozoa with consortia of bacterial strains (E. coli + P. aeruginosa), (E. coli + S. marcescens) and (E. coli + K. pneumoniae), was studied, the results revealed that they were able to cause 100% sperm death within 30 min.
Investigation of the impact of individual bacterial strains and consortia of various bacterial strains on fertility outcome
In order to study the effect of individual bacterial strains and consortia of various bacterial strains on fertility outcome, healthy female Balb/c mice were divided into eight groups comprising 3 mice in each group. These groups were intravaginally inoculated with 101 cfu/20 µl for 10 consecutive days. To study the colonization pattern of these bacterial strains, vaginal lavages were taken from the female mice on every 3rd day viz. day 3, 6 and 9 which showed that the inoculated bacterial strains could efficiently persist in the mouse vagina till day 9.
Colonization studies:
The colonization studies revealed that control group I administered with PBS were culture negative throughout the experiment.
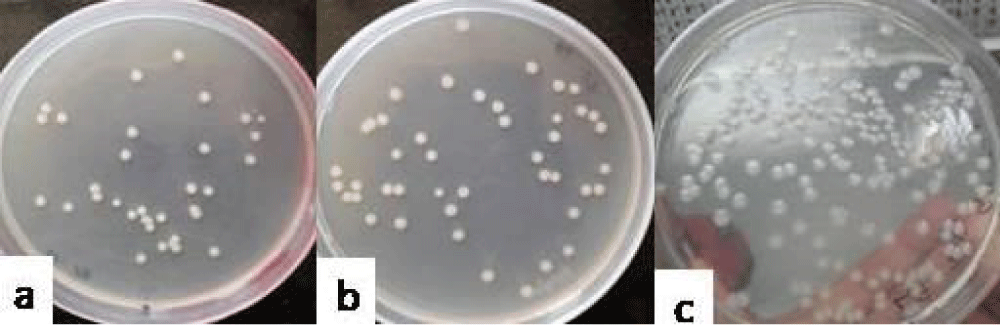
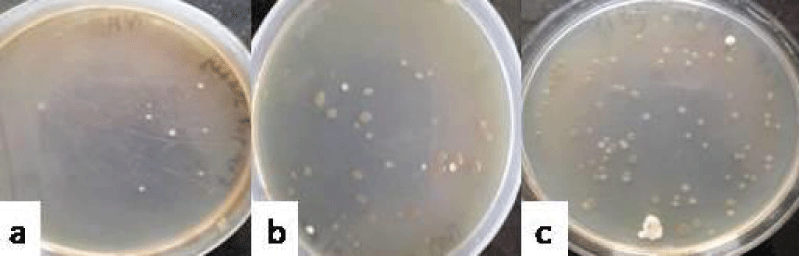
Group II showed that E. coli (E) could be recovered from the vaginal lavages at the counts of 1.8 ± 0.3 (Figure 1a), 2.61 ± 0.39 (Figure 1b) and 3.16 ± 0.27 (Figure 1c) log10cfu ml-1 on day 3, 6 and 9, respectively on nutrient agar. The isolates so obtained from the lavages were confirmed by reisolating on selective media and lavages obtained from all the mice of this group showed green metallic sheen around the colonies when cultured on EMB agar that gives the confirmation of E. coli (Figure 2).
Figure 1: Photograph showing the counts of E. coli in the vaginal lavages of mice obtained on day 3 (a), 6 (b) and 9 (c) administered with 10 doses of 101 cfu/20 µl of E. coli.
Figure 2: Representative photograph of reisolation of E. coli in the vaginal lavages of mice obtained on day 3, 6 and 9 administered with 10 consecutive doses of 101 cfu/20 µl.
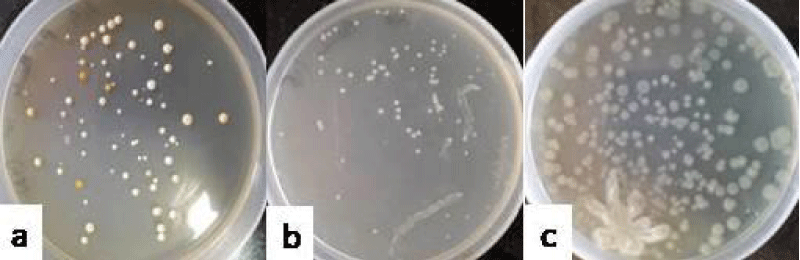
Group III administered with P. aeruginosa could establish itself in mouse vagina at counts of 1.57 ± 0.35 (Figure 3a), 2.78 ± 0.21 (Figure 3b) and 3.49 ± 0.23 (Figure 3c) log 10 cfu ml-1 as observed on sampling days 3, 6 and 9, respectively on nutrient agar. The isolates so obtained from the lavages were confirmed by gram staining and the green colony colour.
Figure 3: Photograph showing the counts of P. aeruginosa in the vaginal lavages of mice obtained on day 3 (a), 6 (b) and 9 (c) administered with 10 doses of 101 cfu/20 µl of P. aeruginosa.
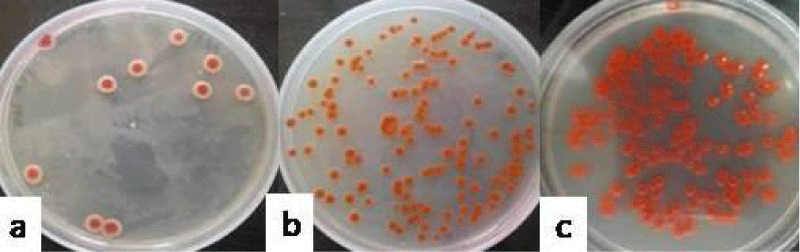
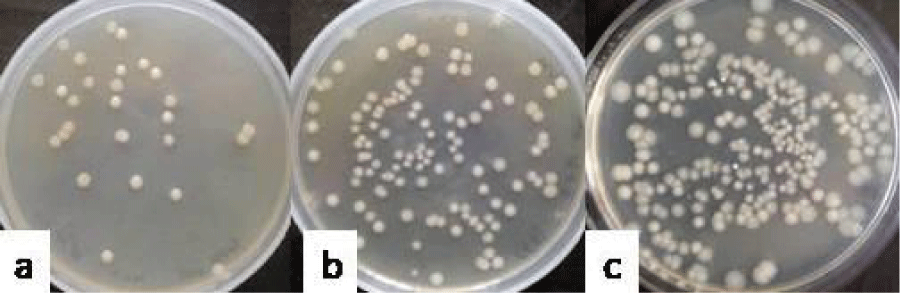
The colonization studies revealed that group IV receiving S. marcescens, showed count of 1.81 ± 0.14 (Figure 4a), 2.68 ± 0.13 (Figure 4b) and 3.67 ± 0.29 (Figure 4c) log10 cfu ml-1 on day 3, 6 and 9, respectively on nutrient agar. The isolates so obtained from the lavages were confirmed by gram staining and the pink colony colour.
Figure 4:Photograph showing the counts of S. marcescens in the vaginal lavages of mice obtained on day 3 (a), 6 (b) and 9 (c) administered with 10 doses of 101 cfu/20 µl of S. marcescens.
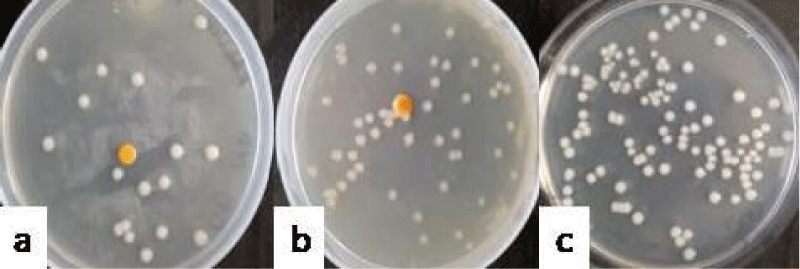
Group V comprising K. pneumoniae showed the count of 1.2 ± 0.18 (Figure 5a), 2.59 ± 0.21 (Figure 5b) and 3.64 ± 0.09 (Figure 5c) log10cfu ml-1 recovered on day 3, 6 and 9, respectively. The lavages obtained from all the mice of this group showed the mucoid, greyish- white, opaque and translucent colonies on nutrient agar.
Figure 5:Photograph showing the counts of K. pneumoniaein the vaginal lavages of mice obtained on day 3 (a), 6 (b) and 9 (c) administered with 10 doses of 101 cfu/20 µl of K. pneumoniae.
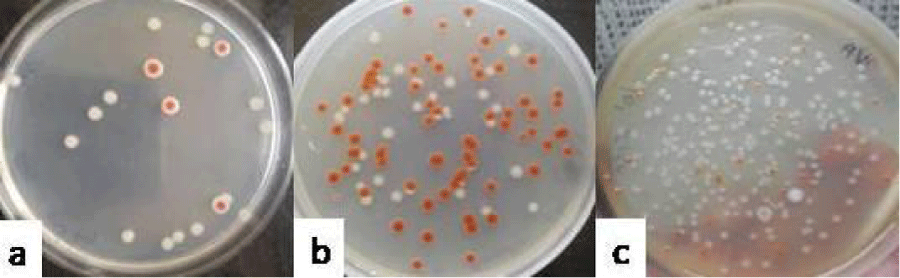
In case of group VI which was administered with the combination of E. coli and P. aeruginosa, the total count of 3.92 ± 0.08 log10 cfu ml-1 (Figure 6a) was recovered on day 3, out of which, the count of E. coli was 3.47 ± 0.14 and count of P. aeruginosa was 3.37 ± 0.24 log10 cfu ml-1. The total count of 4.83 ± 0.14 log10 cfu ml-1 (Figure 6b) could be recovered on day 6, out of which 3.92 ± 0.09 was contributed by E. coli and 4.48 ± 0.10 log10 cfu ml-1 was contributed by P. aeruginosa. On day 9, the total count of 4.99 ± 0.17 log10 cfu ml-1(Figure 6c) could be recovered, out of which count of E. coli was 4.81 ± 0.1 and the count of S.marcescens was 4.71 ± 0.09 log10 cfu ml-1.
Figure 6:Photograph showing the counts of consortia of E+P in the vaginal lavages of mice obtained on day 3 (a), 6 (b) and 9 (c) administered with 10 doses of 101 cfu/20 µl of combination ofEscherichia coli and Pseudomonas aeruginosa.
In case of group VII which was administered with the combination of E. coli (E) and S. marcescens (S), the total count of 4.29 ± 0.07 log10 cfu ml-1 (Figure 7a) was recovered on day 3, out of which, the count of E. coli was 4.18 ± 0.04 and count of S. marcescens was 3.4 ± 0.21 log10 cfu ml-1. The total count of 4.81 ± 0.18 log10 cfu ml-1 (Figure 7b) could be recovered on day 6, out of which 4.35 ± 0.06 was contributed by E. coli and 4.66 ± 0.05 log10 cfu ml-1 was contributed by S. marcescens. On day 9, the total count of 4.97 ± 0.14 log10 cfu ml-1(Figure 7c) could be recovered, out of which count of E. coli was 4.8 ± 0.15 and the count of S. marcescens was 4.41 ± 0.04 log10 cfu ml-1.
Figure 7: Photograph showing the counts of consortia of E+S in the vaginal lavages of mice obtained on day 3 (a), 6 (b) and 9 (c) administered with 10 doses of 101 cfu/20 µl of combination of Escherichia coli and Pseudomonas aeruginosa.
In case of group VIII which was administered with the combination of E. coli (E) and K. pneumoniae (K), the total count of 3.34 ± 0.11 log10 cfu ml-1 (Figure 8a) was recovered on day 3, out of which, the count of E. coli was 3.15 ± 0.06 and count of K. pneumoniae was 3.07 ± 0.05 log10 cfu ml-1. The total count of 4.81 ± 0.08 log10 cfu ml-1 (Figure 8b) could be recovered on day 6, out of which 4.46 ± 0.05 was contributed by E. coli and 4.4 ± 0.17 log10 cfu ml-1 was contributed by K. pneumoniae. On day 9, the total count of 5.15 ± 0.38 log10 cfu ml-1(Figure 8c) could be recovered, out of which count of E. coli was 4.94 ± 0.3 and the count of K. pneumoniae was 4.53 ± 0.12 log10 cfu ml-1.
Figure 8: Photograph showing the counts of consortia of E+K in the vaginal lavages of mice obtained on day 3 (a), 6 (b) and 9 (c) administered with 10 doses of 101 cfu/20 µl of combination of Escherichia coli and Klebsiella pneumoniae.
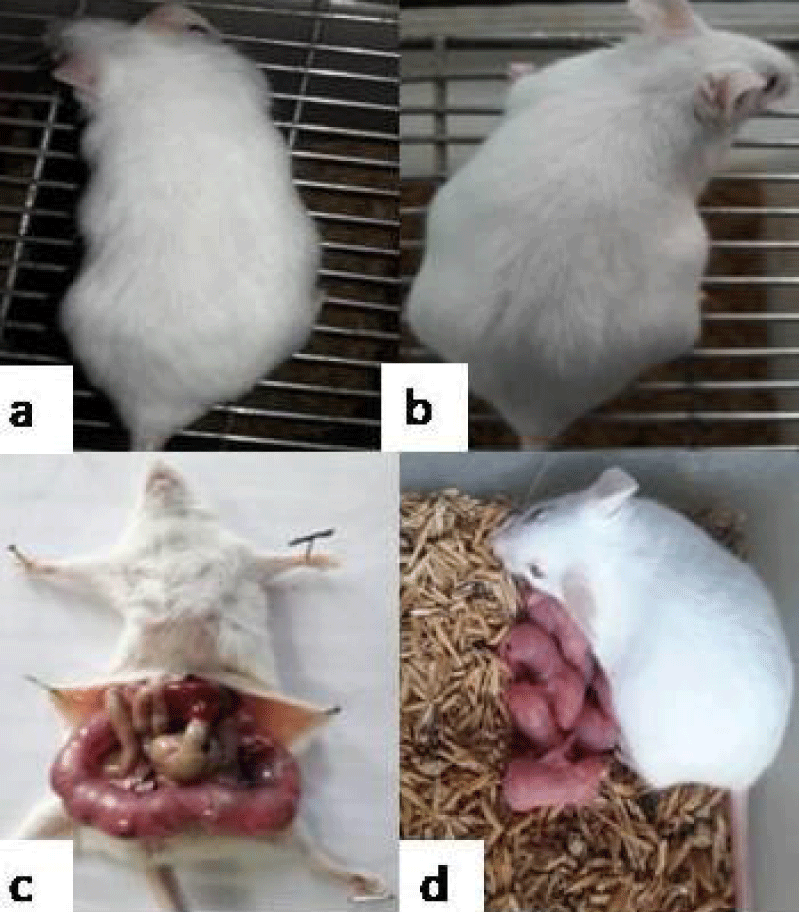
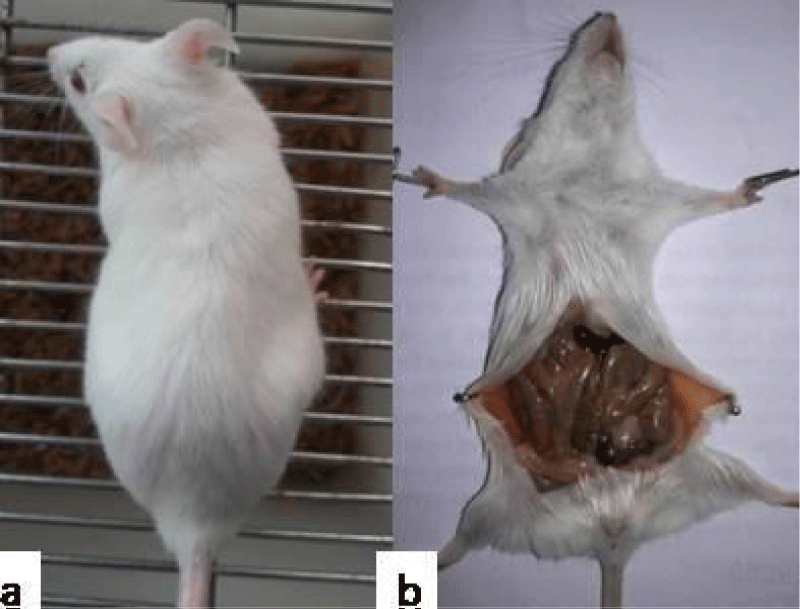
Fertility studies: After 10 consecutive doses, bedding of male mice soiled with faeces and urine was put into the cages of female mice, so as to synchronise them into their oestrous cycle (Whitten effect) on day 11. On day 12, female mice were allowed to mate with proven male mice in a ratio of 2:1. The observation of the presence of copulatory plug that give the confirmation of mating and this day was referred to as day 0 of gestation (GD 0). The control group administered with PBS (group I) and group II, III, IV, V showed consistent weight gain. Pregnancy in group (I-V) was also manifested by visual inspection i.e., abdominal distension and palpation of implantation sites by gestation day (GD) 14. String of pearls could be observed on GD14, upon dissection of mice and mice delivered pups at the end of gestation period i.e., 21 days (Figure 9). On the other hand, in case of group VI, VII, VIII, 100% decrease in fertility was observed (Table 1). All the mice failed to show any pregnancy related changes such as insignificant weight gain, abdominal distension or delivery of pups i.e., they were rendered infertile (Figure 11).
| Table 1: Effect of intravaginal administration of individual bacterial strains and consortia of various bacterialfor ten consecutive days on fertility outcome in Balb/c mice. | |||
| Group | Dose (cfu/20 µl) | Number of treated mice | Fertility outcome |
| Group I (PBS) | - | 3 | 3/3 (100%) |
| Group II | 101 | 3 | 3/3 (100%) |
| Group III | 101 | 3 | 3/3 (100%) |
| Group IV | 101 | 3 | 3/3 (100%) |
| Group V | 101 | 3 | 3/3 (100%) |
| Group VI | 101 | 3 | 0/3 (0%) |
| Group VII | 101 | 3 | 0/3 (0%) |
| Group VIII | 101 | 3 | 0/3 (0%) |
Figure 9: Representative photograph showing pregnancy related changes in mice administered with PBS/individual bacterial strains viz. Normal female (a); Abdominal distention on GD14 (b); String of pearls on GD14 (c); Delivery of pups at the end of gestation period (d).
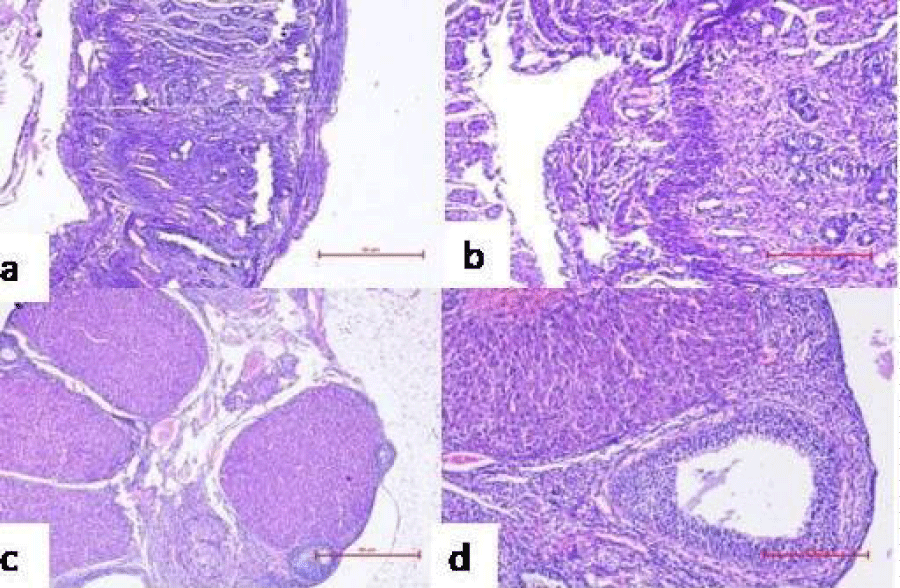
Figure 10: Representative microphotographs of histological examination of reproductive organs of mice administered with PBS/individual bacterial strains for 10 consecutive days showing pregnancy related changes viz. proliferation of endometrium with decidualization of stroma in the uterus at 40X (a) and 100X (b), ovary showing the presence of corpus luteum at 40X (c) and 100X (d).
Figure 11: Representative photograph showing no pregnancy related changes in mice administered with consortia of bacterial strainsviz. Normal female (a); absence of string of pearls on GD14 (b).
Histological investigation of reproductive organs to assess pregnancy related changes
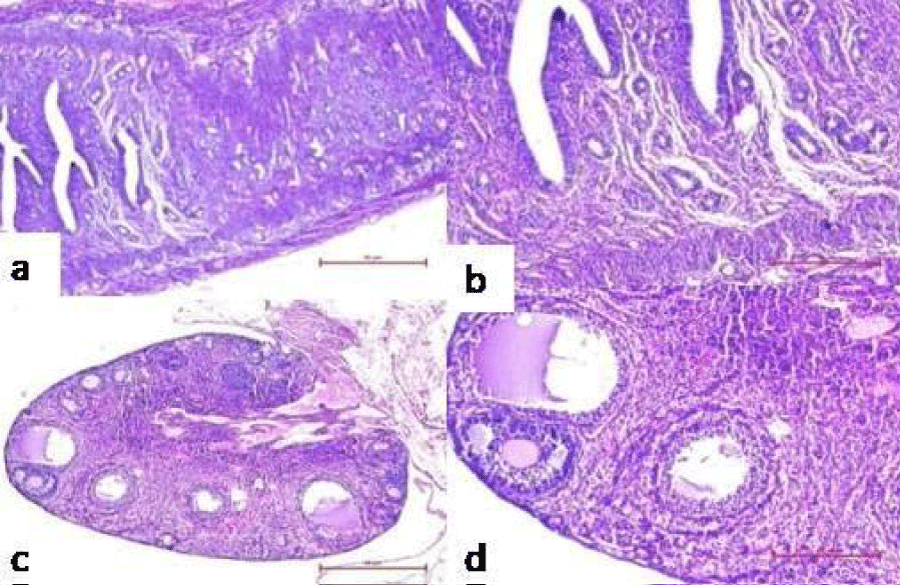
Further, ovary and uterus were observed histologically on GD14 to determine pregnancy related changes in reproductive organs of group I-V. Presence of corpus luteum (an indicative of the pregnancy) induced luteal phase, which persists due to the secretion of progesterone that plays a momentous role in maintaining and establishing pregnancy, was observed in the ovary of mice administered with PBS (Figure 10c). The uterus was also found to have extensive proliferation of uterine glands, increased thickness of endometrium and formation of decidua’s, signifying conception (Figure 10a). However, the uterus of group VI, VII, VIII, did not showed any gross morphologic differences on day 14 post mating (Figure 12a). The primary follicles of the group showed normal appearance and the presence of atretic follicles indicated absence of formation of corpus luteum in ovary (Figure 12c).
Figure 12: Representative microphotographs of histological examination of reproductive organs of mice administered with consortia of bacterial strains showing no pregnancy related changes viz. no decidua’s formation in the uterus at 40X (a) and 100X (b), absence of corpus luteum reveal in the ovary at 40X (c) and 100X (d).
There should be no ambiguity to profess the existence of polymicrobial infections that encompass more than one microbe. In spite of the solemnity of such infections, the research on this topic is still overlooked since formative years. Polymicrobial infections are found to be more severe and rebellious for the therapeutic mediation as compared to the monoculture infections, for this reason, the clinical significance of these infections have been recently given insightful [6]. These infections are challenging to treatment on account of the intricacy in culturing many human associated microbes and moreover targeting multiple etiologic agents at the infection site. In experimental infections, mixture of various aerobic, anaerobic and facultative bacterial species that are leading to synergistic effects, are documented by various studies.
The vaginal ecosystem in female lower genital tract is also a highly complex environment where several aerobic and anaerobic microorganisms coexist in a dynamic balance. The main recurring undesirable organisms are yeasts (Candida albicans, Candida tropicalis, Candida krusei), anaerobic bacteria responsible for vaginosis (Gardnerellavaginalis, Mycoplasma hominis, Atopobiumvaginae, Prevotella spp., Veillonella spp., Mobiluncus spp.), uropathogens (E. coli, Proteus spp., Klebsiella spp., Pseudomonas aeruginosa, Serratia spp.), and sexually transmitted viruses (HIV, Herpes virus) [7]. The positive correlation between presence of uropathogens and reduction in female fertility has been suggested by certain demographic studies but the evidence is not consistent [8,9]. In concordance with predictions, earlier in our laboratory, the role of various individual sperm impairing microorganisms on sperm parameters and female infertility has been elucidated at higher doses. Hence, interest was generated to check the impact of consortia of various sperm impairing microorganisms to investigate how consortia can work together to cause infection when subfertility doses were given. The present study was therefore carried out in a mouse model to elucidate the role of consortia of sperm impairing microorganisms at subfertility doses in fertility outcome.
When the effect of various individual bacterial strains and consortia of bacterial strains grown for 24h was observed on sperm motility, the results showed that individual bacterial strains E.coli, P.aeruginosa, K.pneumoniae and S.marcescens could negatively alter sperm motility at subfertility dose but combinations of these sperm impairing microorganisms at same dose had more significant effect on progressive motility as compared to the individual bacterial strains. Individual bacterial strains viz. E. coli, K.pneumoniae and S.marcescens were found to impair sperm motility via agglutination that showed 40%, 35% and 50% of sperm agglutination in 30 min whereas P. aeruginosa led to impairment of spermatozoa via immobilization and showed 50% sperm immobilization in 30 min. While in case of consortia of (E. coli and S. marcescens) and (E. coli and K. pneumoniae), up to 70% and 60% of sperm agglutination could be observed except in case of consortia of (E. coli and P. aeruginosa) where only 30% sperm agglutination could be observed throughout the incubation time of 30 min which might be due to the presence of sperm immobilization strain i.e. P. aeruginosa that might be responsible for immobilization of maximum spermatozoa and only 30% agglutination could be induced by E.coli. Weinberg, [10] has shown that several combinations of the intestinal organisms given in smaller doses are lethal than the pure cultures themselves, by stressing the role of bacterial synergism in the etiology of acute appendicitis. Carlson, in 1983 [11] also, studied the synergistic effects of S. aureus on mouse mortality and morbidity when combined with a sublethal dose of Candida albicans. The fact underlying these observations is that severity in decrease in sperm motility might be due to synergistic effect of two sperm impairing microorganisms.
In the light of the data obtained in in vitro experiment, it seems coherent to speculate that consortia of sperm impairing microorganisms are important in having severe effect on motility and viability of sperm which in turn may result in infertility. As in vitro conditions do not exclusively reproduce real and pathognomonic conditions of infections of the genital tract, hence, an attempt was made to gain information through in vivo studies using mouse model.
Therefore, in vivo studies were performed to establish the role of individual bacterial strains and consortia of bacterial strains in determining the likelihood of pregnancy. The different groups of mice were intravaginally inoculated with lower count (101 cfu/20 µl) of individual bacterial strains or consortia of bacterial strains. All the microorganisms were capable of establishing themselves in the mouse vagina after ten consecutive doses. It was observed that mice administered with PBS or lower doses of individual bacterial strains showed all the pregnancy related changes whereas mice administered with lower doses of consortia of bacterial strains were rendered infertile. This drastic decline in the fertility rate indicated that these consortia of bacterial strains have some definite involvement in acting synergistically and exerting severe impact on fertility outcome of the studied mice. These results are in concordance with various in vitro and in vivo studies. Kelly, et al. [12] showed the role of pathogenic synergy that occurred in vivo when combinations of sub infective doses of each organism were inoculated that led to copious pus formation. Carlson, et al. [13] also showed the high resistance exhibited by the mice when inoculated with either pathogen alone intraperitoneally while dual infection with the two organisms together resulted in 100% mortality.
Histological examination of the ovary and uterus was conducted to ascertain any pregnancy related changes. These studies were done by taking sections from reproductive tracts of control mice administered with PBS and mice inoculated with 101 cfu/20 µl of individual bacterial strains and consortia of bacterial strains. Histological examination of control mice administered with PBS and mice inoculated with individual strains showed pregnancy related changes such as presence of corpus luteum in ovary and decidua formation in uterus while no such histological changes in the reproductive organs viz. ovaries and uteri of mice inoculated with consortia of bacterial strains were observed.
In conclusion from the results obtained in the present study, it can be concluded that subfertility doses of individual bacterial strains exert less effect on sperm parameters in comparison to consortia of bacterial strains at same doses which showed significantly more impact on these parameters. The colonization of consortia of bacterial strains possessing sperm impairing property resulted in infertility while individual bacterial strains failed to do so. Consequently, this study tends to support with the outlook that the presence of consortia of bacterial strains in female genital tract might be having some synergistic effect at subfertility doses that might play an important role in causing infertility.
Funding
The authors thank Department of Microbiology, Panjab University, Chandigarh for the funding.
- Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016; 535: 94-103. PubMed: https://pubmed.ncbi.nlm.nih.gov/27383984/
- Bakaletz LO. Viral potentiation of bacterial superinfection of the respiratory tract. Trends Microbiol. 1995; 3: 110-114. PubMed: https://pubmed.ncbi.nlm.nih.gov/7773588/
- Kuramitsu HK, He X, Lux R, Anderson MH, Shi W. Interspecies interactions within oral microbial communities. Microbiol Mol Biol Rev. 2007; 71: 653-670. PubMed: https://pubmed.ncbi.nlm.nih.gov/18063722/
- Brogden KA, Guthmiller JM. Polymicrobial diseases, a concept whose time has come. ASM News-American Society for Microbiology. 2003; 69: 69-73. PubMed: https://pubmed.ncbi.nlm.nih.gov/21735561/
- Emmens CW. The motility and viability of rabbit spermatozoa at different hydrogen ion concentrations. J Physiol. 1947: 471-481. PubMed: https://pubmed.ncbi.nlm.nih.gov/16991777/
- Korgaonkar A, Trivedi U, Rumbaugh KP, Whiteley M. Community surveillance enhances Pseudomonas aeruginosa virulence during polymicrobial infection. Proc Natl Acad Sci USA. 2013; 110: 1059-1064. PubMed: https://pubmed.ncbi.nlm.nih.gov/23277552/
- Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, et al. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG. 2011; 118: 533-549. PubMed: https://pubmed.ncbi.nlm.nih.gov/21251190/
- Anchanadevi C. Molecular characterization of pathogens isolated from endometrial samples of female infertility cases. Int J Recent Sci Res. 2013; 4: 614-618.
- Campisciano G, Florian F, D'Eustacchio A, Stanković D, Ricci G, et al. Subclinical alteration of the cervical–vaginal microbiome in women with idiopathic infertility. J Cellular Physiol. 2017; 232: 1681-1688. PubMed: https://pubmed.ncbi.nlm.nih.gov/28098358/
- Weinberg M, Prévot AR, Davesne J, Renard C. The microbial flora of appendicitis. Comptes Rendus de la Sociiti de Biologie, Paris. 1928; 98: 749.
- Carlson E. Effect of strain of Staphylococcus aureus on synergism with Candida albicans resulting in mouse mortality and morbidity. Infection and Immunity.1983; 42: 285-292. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC264556/
- Kelly MJ. The quantitative and histological demonstration of pathogenic synergy between Escherichia coli and Bacteroides fragilis in guinea-pig wounds. J Medi Microbiol. 1978; 11: 513-523. PubMed: https://pubmed.ncbi.nlm.nih.gov/364068/
- Carlson E. Synergistic effect of Candida albicans and Staphylococcus aureus on mouse mortality. Infection and immunity.1982; 38: 921-924. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC347837/