More Information
Submitted: 26 October 2020 | Approved: 30 November 2020 | Published: 01 December 2020
How to cite this article: Elnashar AT, Sabry M. Chronic endometritis in in vitro fertilization failure patients. Clin J Obstet Gynecol. 2020; 3: 175-181.
DOI: 10.29328/journal.cjog.1001073
ORCiD: orcid.org/0000-0003-1079-2112
Copyright License: © 2020 Elnashar AT, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: CD138; Syndecan-1; Chronic endometritis; IVF
Chronic endometritis in in vitro fertilization failure patients
Afaf T Elnashar1* and Mohamed Sabry2
1Pathology Department, Sohag University, Egypt
2Obstetrics and Gynecology Department, Sohag University, Egypt
*Address for Correspondence: Afaf T Elnashar, Pathology Department, Sohag University, Egypt, Tel: 01064641550; Email: [email protected]
Introduction: Chronic endometritis (CE) is a common cause of infertility in asymptomatic patients and its diagnosis and treatments improved assisted reproduction technique outcome in most of the specialized centers. Diagnosis of CE in endometrial biopsy by Hematoxylin and Eosin (H&E) stain is hard to identify chronic inflammatory cells from the stroma and the use of plasma cells-specific stains is helpful.
Aim of the work: Evaluation of the use of CD138 in the identification of plasma cells in endometrial biopsy of patients with previous IVF trial failure.
Material and methods: Hysteroscopic and curettage endometrial biopsies from fifty-five females with previous IVF trial failure were stained with H&E and CD138 immunostaining for detection of plasma cells.
Results: Plasma cells were identified in 52.7% of cases by H&E and in 6/55 by CD138 immunostaining. CD138 is more sensitive in detecting plasma cells in endometrial biopsy than H&E stain. There was a significant statistical correlation between CE and abnormal uterine bleeding, abortion and primary infertility (p > 0.5).
Conclusion: Diagnosis of CE is helpful in infertility patients with IVF trial failure to improve the outcome of the maneuver. CD138 is more sensitive for plasma cells specially in endometrial biopsies than H&E.
In spite of the fact that uterine abnormalities are considered to have a major impact on the possibilities to conceive through IVF, conventional infertility investigations, including ultrasound and hysterosalpingography (HSG) may miss subtle intrauterine lesions. After hysteroscopy maneuver before performing IVF, the incidence of undiagnosed intrauterine lesions has been demonstrated to be at the range of 11% - 45% [1]. One of these abnormalities is chronic endometritis (CE) that is often asymptomatic or can be accompanied by non-specific symptoms. The use of histopathology in detection of plasma cells in the endometrial curettage is essential for the diagnosis of CE [2].
Chronic endometritis may interfere with receptivity of the endometrium and therefore, leads to infertility because CE is characterized by an abnormal pattern of lymphocytic infiltration and consequently, an abnormal endometrial microenvironment [3]. In a study at 2014, it was demonstrated that CE is a frequent finding in females with repeated abortions, and consequently, women who took antibiotic therapy had a significantly higher rate of successful pregnancies compared to those without treatment [4]. Mainwile, CE was a pathological diagnosis in about 30% of females with repeated implantation failure at IVF and those that were diagnosed as CE had about (11.5%) lower rates of implantation after an IVF cycle [5].
On the other hand, Kasius, et al reported a minimal clinical implication of CE on fertility after IVF and they documented CE in only (2%) of asymptomatic women with infertility. They concluded that pregnancy rate after IVF was not affected by CE [6].
Syndecan-1 or CD138 is a well-known trans-membrane heparan sulfate proteoglycan family member, which acts as a receptor at extracellular matrix and is involved in different cellular functions, mainly cell-cell and cell-matrix adhesion. Expression of CD138 is observed normally on the surface of mature epithelial cells; however, stromal expression also was detected in developing tissues. In the hematopoietic system, expression of CD138 is thought to be restricted to normal and malignant plasma cells. Based on messenger RNA studies, high levels of CD138 was restricted to the precursor B-cell and plasma cell stages of B-cell differentiation [7].
B-cells constituted about <1% of all leukocytes in normal endometrium. B-cell infiltration is rarely detected, in the functional layer of the endometrium but is found mainly in the basal layer, as central cells in the lymphocyte aggregates surrounded by macrophages and many CD8+ T-cells. In chronic non-specific endometritis, a large number of B-cells focally, infiltrates both the endometrial stroma and glandular epithelium [8].
Accumulating evidence documented the efficiency of antibiotic oral therapy to eliminate endometrial stromal plasma cells in patients with CE. On the other hand, it is unclear to the investigators that how antibiotic treatment changes endometrial microbial profiling in CE. Several studies documented the abnormal expression of many pro-inflammatory chemical mediators involved in B-cell extravasation in CE. Selectin plays an important role in tethering/rolling of circulating B-cells on endothelial cells. While Selectin E is not detected in normal endometrium, in CE endometrial vascular endothelial cells express this B-cell adhesion molecule. Moreover, endometrial microvascular endothelial cells specifically expressed CXCL-13 in CE, which is a chemo-attractant that activates the adhesion molecules on B-cells and endothelial cells. Endometrial glandular epithelial cells with CE also expressed CXCL-1, a chemokine involved in B-cell migration [9]. All these findings suggest that local microbial infection in the endometrium stimulates the immune responses, which potentially retrieve circulating B-cells into the endometrial stromal the glandular areas. The migrating B-cells may differentiate into plasma cells within the endometrial stroma. It was found that endometrial stromal plasma cells express multiple immunoglobulin subclasses (IgM, IgA1, IgA2, IgG1, and IgG2) with a predominant IgG2. These excessive mucosal antibodies affect the process of embryo implantation negatively [10].
Endometrial micro polyposis was reported to be associated with endometrial stromal edema, thickening, and peri-glandular hyperemia noticed with CE. These lesions cannot be detected by ultrasonography or hysterosalpingography but can be diagnosed by fluid hysteroscopy. Hysteroscopy therefore has an important role in the diagnosis of CE in Gynecology and Obstetrics practice. On the contrary, local bacterial examination alone is unlikely to be useful in the diagnosis of CE [11].
A small number of plasma cells in the endometrial curettage biopsy are difficult to be identified in the H&E-stained sections. In addition, the histological changes in the proliferative and secretory phases of endometrium can also interfere with the detection of plasma cells and the diagnosis of CE. During late menstruation or early proliferative endometrium, significant lymphocytes infiltrations, mitosis in the stroma, and plasma cell-like stromal cells, appear in the endometrial tissue. These can simulate plasma cells in the endometrial tissue and interfere with the diagnosis of CE [12].
To study the use of immunohistochemical expression of CD138 in the diagnosis of chronic endometritis (CE) which is considered as a risk factor for assisted reproduction (IVF) failure patients.
In order to uncover the controversial role of CE in IVF failure women, we evaluated the incidence of CE by both hysteroscopy and histopathology in 55 women who experienced IVF-failure (failure to get pregnancy). The study protocol was accepted by the Sohag Medical ethical committee and every female assigned a written consent for the maneuvers (both hysteroscopy and endometrial biopsy that were performed as a part of workup for IVF-ET second trial). Those cases who had been diagnosed with CE in the study were given the appropriate antimicrobial drugs and were given a period of 6 months to 1 year before starting another trial of IVF. Specific exclusion criteria of the study cases including:
1. Females with intrauterin contraceptive devices, (as it is characterized by prolonged plasma cell accumulation even after their removal from the uterine cavity).
2. Endometriosis.
3. Post-gestational long-term retention of products of conception.
4. Acute suppurative endometritis (recognized as neutrophil invasion and micro abscess formation in the endometrium).
5. Uterine and/or cervical tumors.
6. Previous recent treatment with chemotherapy.
7. Specific disease as hypothyroidism, tuberculosis, diabetes, liver or renal chronic diseases.
After hysteroscopy, endometrial biopsies were collected from 55 cases of IVF trial failure females, specifically from the endometrial micro polyposis areas. It was fixed immediately by immersion in 10% neutral formaldehyde for fixation. After ethanol gradient dehydration, the endometrial tissues were cleared with Xylene, immersed in paraffin for embedding, and 4 μm serial sections were cut. All paraffin blocks were stained with routine H&E and Geimsa stains for detection of plasma cells and diagnosing CE. (Geimsa stain was used to confirm the diagnosis of CE and the presence of plasma cell detected by H&E as the plasma cells were stained red in color with Geimsa stain so were easily detected). Paraffin-embedded, endometrial tissue blocks were cut and mounted on 3-aminopropyl- trethoxy silane-coated slides and stained using Syndecan-1 (monoclonal mouse antihuman CD138; Clone M115, Dako) at a dilution 1/50 for 2 H, at room temperature). The immunostained slides were scored for the presence of membranous immunostaining of plasma cells using light microscopy, in at least 10 HPF. Each section was graded as Negative: when no plasma cells stained with CD138 (+) when <5 plasma cells were detected, (++) when 5-10 plasma cells were detected and (+++) when >10 plasma cells were stained [13]. The secondary histologic features of CE like gland architectural irregularity, spindling of the stroma, stromal edema and/or hemorrhage with the presence of plasma cells were mentioned. All immunohistochemical and H&E findings were statistically analyzed using SPSS software version 14 and Fischer’s exact tests. Value of p < 0.05 was considered significant [14].
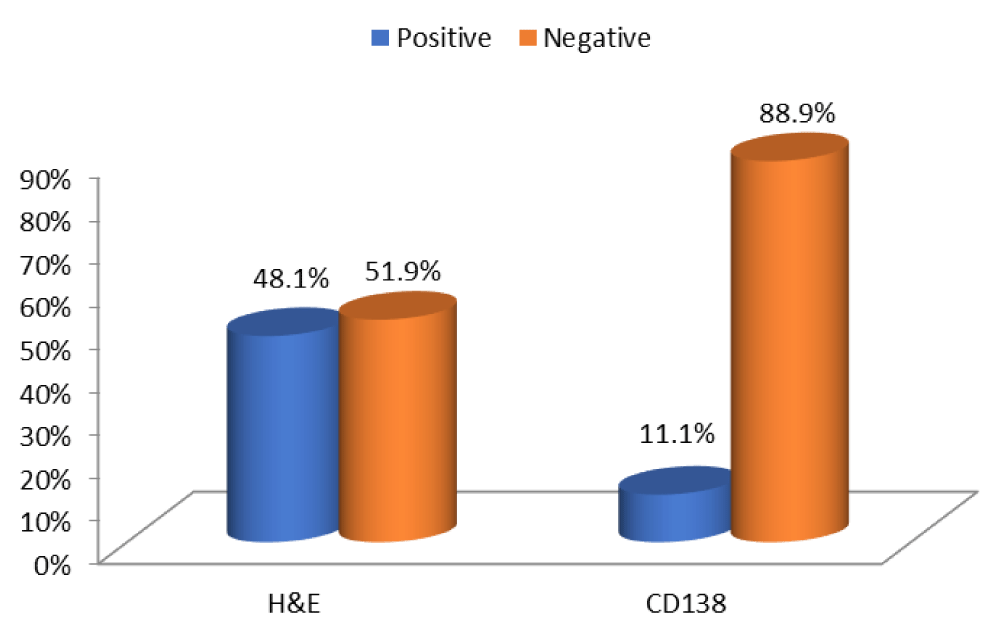
Fifty-five women with a history of previous IVF failure were included in this study with age range 18-45 years with mean age 31.5 years. Twenty-seven cases of diagnosed as primary infertility while 28 cases complained of secondary infertility. Proliferative endometrium was seen in 32 cases and secretory endometrium was seen in 23 cases. Seven cases had previous abortions, and 27 cases complained of prolonged abnormal uterine bleeding. CE was diagnosed in 29/55, (52.7%) by H&E and Geimsa stains and in 6 cases (10.9%) by CD138 immunostaining. A previous history of abnormal uterine bleeding episodes, abortion and primary infertility due to fallopian tube obstruction were statistically correlated with CE (p < 0.05). CD138 immunostaining expression had no statistically significant correlation with age of the patient (p < 0.7), secondary infertility (p < 0.05), or the type of endometrium either proliferative of secretory (p < 0.6). CD138 is more sensitive test (sensitivity 66.7%) but less specific for plasma cells (specificity 48.9%) while routine H&E staining was detected to be less sensitive than CD138 (sensitivity 55.7%) and equal to CD138 as specific for plasma cells detection (specificity 50%). The use of H&E together with Geimsa stain is sensitive and specific for detection of plasma cells in endometrial biopsy as CD138 (Tables 1,2 & Figures 1,2).
| Table 1: The clinicopathological characteristics of the study groups. | ||||
| NO | CE + with H&E | CE + with CD138 | p value | |
| Age range | 20-45 | |||
| 1ry Infertility | 27 | 13 | 3 | 0.03 |
| 2dry infertility | 28 | 16 | 3 | 0.06 |
| Tubal factor | 19 | 13 | 0.005 | |
| Previous miscarriage | 7 | 4 | 0.005 | |
| AUB | 27 | 16 | 0.002 | |
| Proliferative E | 32 | 16 | 3 | |
| Secretory E | 23 | 13 | 3 | |
| CE | 29(52.7%) | 6/55(12%) | ||
| Table 2: The sensitivity and specificity of both H&E and CD128 in detection of plasma cells in CE. | ||
| H&E | CD138 | |
| Sensitivity | 55.7% | 66.7% |
| Specificity | 50% | 48.9% |
| +ve predictive value | 52.7% | 13.8% |
| -ve predictive value | 89.7% | 92.3% |
Figure 1: The representation of H&E and CD138 positive plasma cells in 1ry infertility cases.
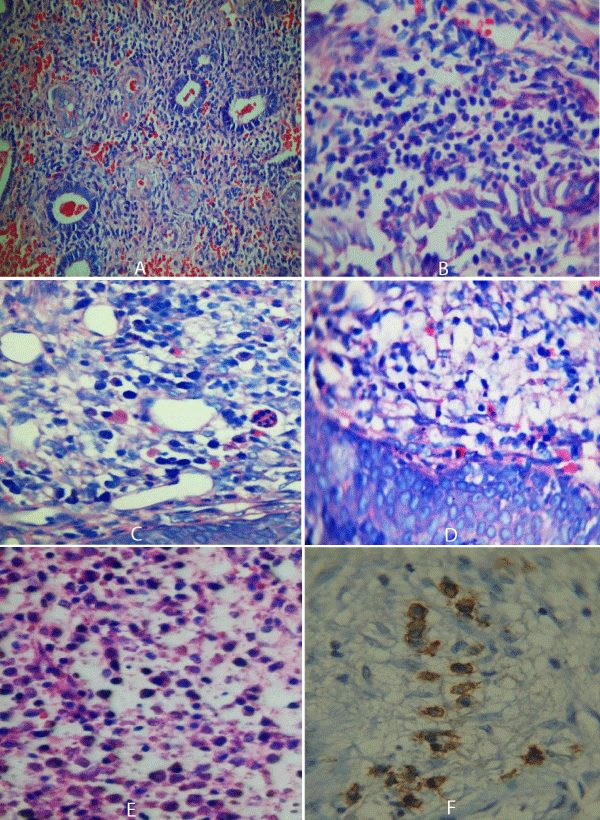
Figure 2: A: Proliferative endometrium with CE (H&E) X 40). B: Endometrial infiltrate with B-cells stained by Geimsa stain (X 100). C: D: CE with plasma cells stained red by Geimsa stain (X 100). E: Plasma cells stained with H&E in a case with previous abortion (X 200). F: Plasma cells membranous immunostaining by CD138 (X 200).
Infertility is a relatively common gynecological problem. Relevant studies have shown that the incidence of chronic endometritis(CE) in infertile patients ranges from 0.2% – 46% [15].
Chronic endometritis (CE) is a local non-specific inflammatory disease characterized by abnormal plasma cells infiltration in the endometrial stromal areas. In the present study CE was detected by H&E in 52.7% (29/55) and in 12% by CD138 of the studied cases that experienced implantation failure after IVF trial. There was a significant statistical correlation between CE and specific risk factors as abnormal uterine bleeding, previous abortion, and primary infertility caused by tubal factor (p > 0.5).
Recent studies have disclosed the association between CE and reproductive failure. Chronic endometritis was identified in 30% of the patients with repeated implantation failure after in vitro fertilization-embryo transfer, in 28% of the patients with unexplained infertility, and in 12% of the patients with unexplained recurrent miscarriages [16-18].
An earlier study on the underlying causative agents in CE found that the microorganisms detected more frequently in the endometrium with CE are common bacteria [Streptococcus species (27%), Escherichia coli (11%), Enterococcus faecalis (14%)] and mycoplasma species (Mycoplasma Genitalium (15%) and Ureaplasma Urealyticum (11%). The bacterial flora in the endometrial tissue culture was inconsistent with those in the vaginal or endocervical swab culture in patients with CE [19].
In the present study a mixture of bacterial (Streptococci species), Fungal (Candida Albicans) and viral (HPV) were detected in the cervical swab examination of the studied cases but none of these showed a statistically significant correlation with CE. A recent study demonstrated that CE is a common finding in women complaining of repeated unexplained implantation failure. The reproductive outcome at IVF was significantly improved in those patients in whom antibiotic treatment was able to normalize both hysteroscopic and histologic endometrial pattern [20].
In agreement with our results, it was suggested in a recent study at 2016 that a previous prolonged menstrual bleeding episode, an abortion history or fallopian tube obstruction were associated with the pathogenesis of chronic endometritis and were independent risk factors of CE. They have shown that among infertile patients, the prevalence of CE was 27.96% and among patients who received assisted reproductive technology, the pregnancy rate in the control group (31.3%) was significantly higher than that of the chronic endometritis group (7.7%) (p = 0.017 < 0.05), indicating that chronic endometritis may be one of the causes of infertility [12]. Chronic endometritis was detected in 14% of women with repeated implantation failure and in 27% in women with recurrent pregnancy losses in a more recent study at 2016 [21].
Although the association between CE (plasma cells infiltration of the endometrium) and abnormal uterine bleeding still remains to be established, there was a statistically significant correlation between CE and abnormal uterine bleeding (p > 0.5) in our study. It was demonstrated that endometrium with CE uniquely expresses the chemokines CXCL-1, CXCL-13 and adhesion molecules Selectin E, implicating that local B-cells are recruited from endometrial microcirculation and differentiate in-situ into plasma cells. Such unusual leucocyte composition in CE may disrupt the integrity of the epithelial lining and cause endometrial shedding resulting in abnormal uterine bleeding (AUB) [17].
In the present study, CE was detected in 52.7% of cases which is considered to be high rate compared to a cohort study of 395 cases of infertility with a history of two or more early pregnancy losses, CE was found in 9% of cases and the cure rate was 100% after a course of antibiotics and subsequent livebirth rate was 88% [22]. Another study found that CE was detected in 30.3% of patients with recurrent implantation failure and they concluded that recurrent implantation failure warrants investigations on CE as a contributing factor [16]. This discrepancy of CE incidence rate can be explained on the bases of a lower socioeconomic standard in most of the developing countries including Egypt that did not provide regular medical surveillance programs for early diagnosis and treatment of CE especially in asymptomatic infertile women.
In the present study CD138 showed sensitivity and specificity for detection of plasma cells in endometrial biopsy of 66.7% and 48.9% respectively in agreement with a similar study that evaluated Syndecan-1 (CD138) immunohistochemical staining of endometrial biopsies in 107 women with repeated pregnancy losses. They concluded that CD138 provided increased sensitivity for chronic endometritis diagnosis compared to H&E staining [22].
On the other hand, a prevalence of CE in 606 patients with an adequate biopsy was 2.8% in an earlier study with a cumulative live birth rate (including spontaneous pregnancies) did not significantly differ between patients with or without CE (76% vs. 54%) (2).
Diagnosis and treatment of CE significantly improves implantation rate in patients undergoing IVF-ET (in vitro Fertilization and Embryo Transfer) in a study that concluded CE to be found in 40.7% (11/27) of women [23]. While in an earlier study, CD138-positive plasma cells were detected in 18 cases of CE while none of the 25 cases of abnormal uterine bleeding showed immunostaining for CD138 [24]. Kannar, et al diagnosed CE in 3/50 cases by H&E and in 22/50 cases by immunohistochemistry technique [25], while in Hartman, et al.
plasma cells were stained by H&E in 29/100 and by CD138 in 90/100 cases of CE [26]. The different and variable correlation between CE and infertility risk factors as abnormal uterine bleeding in the studies necessitated a further work with larger groups of patients using cohort studies.
Syndecan-1 (CD138) is a sensitive immunohistochemical marker for detection of plasma cells in CE than the routine H&E and Geimsa stains specially in asymptomatic infertility patients. Larger cohort studies are needed in the future to evaluate the role of CE in patients with IVF and implantation failure to improve the outcome of these techniques.
- Fatemi HM, Kasius JC, Timmermans A, van Disseldorp J, Fauser BC, et al. Prevalence of unsuspected uterine cavity abnormalities diagnosed by office hysteroscopy prior to in vitro fertilization. Hum Reprod. 2010; 25: 1959-1965. PubMed: https://pubmed.ncbi.nlm.nih.gov/20570971/
- Kasius JC, Fatemi HM, Bourgain C, Sie-Go DMD, Eijkemans RJC, et al. The impact of chronic endometritis on reproductive outcome. Fertil Steril. 2011; 96: 1451-1456. PubMed: https://pubmed.ncbi.nlm.nih.gov/22019126/
- Matteo M, Cicinelli E, Greco P, Massenzio F, Baldini D, et al. Abnormal pattern of lymphocyte subpopulations in the endometrium of infertile women with chronic endometritis. Am J Reprod Immunol. 2009; 61: 322-329. PubMed: https://pubmed.ncbi.nlm.nih.gov/19341383/
- Cicinelli E, Matteo M, Tinelli R, Pinto V, Marinaccio M, et al. Chronic endometritis due to common bacteria is prevalent in women with recurrent miscarriage as confirmed by improved pregnancy outcome after antibiotic treatment. Reprod Sci. 2014; 21: 640-647. PubMed: https://pubmed.ncbi.nlm.nih.gov/24177713/
- Quaas A, Dokras A. Diagnosis and treatment of unexplained infertility. Rev Obstet Gynecol. 2008; 1: 69-76. PubMed: https://pubmed.ncbi.nlm.nih.gov/18769664/
- Kasius JC, Broekmans FJM, Sie-Go DM, Bourgain C, Eijkemans MJC, et al. The reliability of the histological diagnosis of endometritis in asymptomatic IVF cases: a multicenter observer study. Hum Reprod. 2012; 27: 153-158. PubMed: https://pubmed.ncbi.nlm.nih.gov/22025228/
- O’Connell FP, Pinkus JL, Pinkus GS. CD138 (Syndecan-1) A plasma cell marker, immunohistochemical profile in hematopoietic and non- hematopoietic neoplasms. Am J Clin Pathol. 2004; 121: 254-263. https://pubmed.ncbi.nlm.nih.gov/14983940/
- Kitaya K, Yamada H. Pathophysiological roles of chemokines in human reproduction: an overview. Am J Reprod Immunol. 2011; 65: 449–459. PubMed: https://pubmed.ncbi.nlm.nih.gov/21087337/
- Kitaya K and Yasuo T. Aberrant expression of Selectin E, CXCL1, and CXCL13 in chronic endometritis. Mod Pathol. 2010; 23:1136–1146. PubMed: https://pubmed.ncbi.nlm.nih.gov/20495539/
- Kitaya K, Matsubayashi H, Yamaguchi K, Nishiyama R, Takaya Y, et al. Chronic endometritis: Potential cause of infertility and obstetric and neonatal complications. A J Reproductive Immunol. 2016; 75: 13-22. PubMed: https://pubmed.ncbi.nlm.nih.gov/26478517/
- Cicinelli E, Resta L, Nicoletti R, Zappimbulso V, Tartagni M, et al. Endometrial micro polyps at fluid hysteroscopy suggest the existence of chronic endometritis. Hum Reprod. 2005; 20: 1386– 1389. PubMed: https://pubmed.ncbi.nlm.nih.gov/15734762/
- Chen YQ, Fang RL, Luo YN, Luo CQ. Analysis of the diagnostic value of CD138 for chronic endometritis, the risk factors for the pathogenesis of chronic endometritis and the effects of chronic endometritis in pregnancy: A cohort study. BMC Women’s Health. 2016; 16: 60. PubMed: https://pubmed.ncbi.nlm.nih.gov/27596852/
- Al-Quran SZ, Yang L, Magill JM, Braylan RC, Douglas-Nikitin VK. Assessment of bone marrow plasma cell infiltrates in multiple myeloma: the added value of CD138 immunohistochemistry. Human Pathol. 2007; 38: 1779–1787. PubMed: https://pubmed.ncbi.nlm.nih.gov/17714757/
- Florkowski CM. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: communicating the performance of diagnostic tests. Clin Biochem Rev. 2008; 29: S83-S87. PubMed: https://pubmed.ncbi.nlm.nih.gov/18852864/
- Carvalho FM, Aguiar FN, Tomioka R, de Oliveira RM, Frantz N, et al. Functional endometrial polyps in infertile asymptomatic patients: a possible evolution of vascular changes secondary to endometritis. Eur J Obstet Gynecol Reprod Biol. 2013; 170: 152–156. PubMed: https://pubmed.ncbi.nlm.nih.gov/23773528/
- Johnston-MacAnanny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM, et al. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2010; 93: 437-441. PubMed: https://pubmed.ncbi.nlm.nih.gov/19217098/
- Kitaya K. Prevalence of chronic endometritis in recurrent miscarriages. Fertil Steril. 2011; 95: 1156-1158. PubMed: https://pubmed.ncbi.nlm.nih.gov/21030015/
- McQueen DB, Benardi LA, Stephenson MD. Chronic endometritis in women with recurrent early pregnancy lose and/ or fetal demise. Fertil Steril. 2014; 101: 1026-1030. PubMed: https://pubmed.ncbi.nlm.nih.gov/24462055/
- Cicinelli E, De Ziegler D, Nicoletti R, Colafiglio G, Saliani N, et al. Chronic endometritis: correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil Steril. 2008; 89: 677–684. PubMed: https://pubmed.ncbi.nlm.nih.gov/17531993/
- Cicinelli F, Matteo M, Tinelli R, Lepera A, Alfonso R, et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Human Reprodu. 2015; 30: 323-330. PubMed: https://pubmed.ncbi.nlm.nih.gov/25385744/
- Bouet PE, El-Hachem H, Monceau E, Gariepy G, Kadoch IJ, et al. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: Prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil Steril. 2016; 105: 106-110. PubMed: https://pubmed.ncbi.nlm.nih.gov/26456229/
- McQueen DB, Perfello CO, Hazard FK, Lathi RB. Pregnancy outcomes in women with chronic endometritis and recurrent pregnancy loss. Fertil Steril. 2015; 104: 927-931. PubMed: https://pubmed.ncbi.nlm.nih.gov/26207958/
- Lewis EI, Brower M, Shamonki M. Treatment of chronic endometritis in women with implantation failure improves implantation in subsequent embryo transfers. Fertil Steril. 2013; 100: S390.
- Bayer-Garner IB, Korourian S. Plasma cells in chronic endometritis are easily identified when stained with syndecan-1. Modern Pathol. 2001; 14: 877-879. PubMed: https://pubmed.ncbi.nlm.nih.gov/11557783/
- Kannar V, Lingaiah HKM, Sunita V. Evaluation of endometrium for chronic endometritis by using syndecan-1 in abnormal uterine bleeding. J Laboratory Physician. 2012; 4: 69-73. PubMed: https://pubmed.ncbi.nlm.nih.gov/23440678/
- Hartman SK, Symons WA, Yeh T. Chronic endometritis: How many plasma cells does it take to make the diagnosis? FASEB. 2011; 25: 1002-1013.