More Information
Submitted: 21 September 2020 | Approved: 27 November 2020 | Published: 30 November 2020
How to cite this article: Hazari KS, Paulose L, Kurien N, Mohammad H, Elgergawi TFA, et al. Clinical characteristics, management, maternal and neonatal outcome among seven severe and critically ill pregnant women with COVID-19 pneumonia. Clin J Obstet Gynecol. 2020; 3: 158-166.
DOI: 10.29328/journal.cjog.1001071
Copyright License: © 2020 Hazari KS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Adult respiratory syndrome (ARDS); Novel coronavirus disease 2019 (COVID-19); Pregnancy; Cytokine storm; Maternal mortality and morbidity
Clinical characteristics, management, maternal and neonatal outcome among seven severe and critically ill pregnant women with COVID-19 pneumonia
Komal Sundeep Hazari1, Litty Paulose1, Nimmi Kurien2, Hozaifah Mohammad2, Taghrid Faek A Elgergawi1, Atif Bashir E Fazari1,3* and Amar Hassan4
1Latifa Women and Children Hospital, Dubai Health Authority, Dubai, United Arab Emirates
2Rashid Hospital, Dubai Health Authority, Dubai, United Arab Emirates
3University of Medical Sciences and Technology, Faculty of Medicine, Khartoum, Sudan
4Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, United Arab Emirates
*Address for Correspondence: Atif Fazari, Latifa Hospital, DHA Dubai, UAE, University of Medical Sciences & Technology, Faculty of Medicine, Khartoum, Sudan, Email: [email protected]
Pneumonia caused by the Novel coronavirus disease 2019 (COVID-19) is a highly infectious disease and the ongoing outbreak has been declared as a Pandemic by the World health organization. Pneumonia is a serious disease in pregnancy and requires prompt attention. Viral pneumonia has higher morbidity and mortality compared to bacterial pneumonia in pregnancy. All efforts are well exerted to understand the newly emerged disease features but still some areas are gray.
The treatment is primarily supportive with antivirals, steroids, anticoagulation and antibiotics for secondary bacterial infection. Severe cases require intensive care monitoring with oxygen support, mechanical ventilation. Investigational therapies include convalescent plasma, cytokine release inhibitors and other immunomodulatory agents like interferons. The mortality appears driven by the presence of severe Adult Respiratory Syndrome (ARDS) and organs failure.
COVID pandemic is a challenging and stressful socio-economic situation with widespread fear of infection, disease and death. In the specialty of obstetrics and gynecology, studies are being conducted to ascertain the manifestation of disease in pregnant women and the fetal outcome.
The aim of our case series is to describe the demographics, clinical characteristics, laboratory and radiological findings, feto- maternal outcome of severe and critical COVID pneumonia in pregnant women in Latifa Hospital.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the novel coronavirus that first out broke in Wuhan, China which causes coronavirus disease 2019 (COVID-19). Pneumonia caused by the Novel coronavirus disease 2019 (COVID-19) is a highly infectious disease and the ongoing outbreak has been declared as a Pandemic by the World health organization [1,2].
COVID-19 has now been reported from 213 countries and territories around the world, affecting over 10 million people resulting in more than five hundred thousand deaths as of July 2020 [2]. In the United Arab Emirates, a total of 55,848 laboratory-confirmed COVID-19 cases were reported resulting in 335 deaths as per the latest statistics.
Coronaviruses are enveloped single -stranded RNA viruses belonging to the same subgenus as the severe acute respiratory syndrome (SARS) virus which can cause respiratory, enteric, hepatic and neurological diseases.
The Coronavirus Study Group of the International Committee on Taxonomy of Viruses has proposed that this virus be designated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. The incubation period for COVID-19 is usually within 14 days following exposure, with the commonest symptoms being cough, fever, myalgias and headache. The spectrum of disease ranges from asymptomatic cases to severe and critical disease with Pneumonia being the most common serious manifestation. Acute respiratory distress syndrome (ARDS) leading to respiratory failure is one of the major complications of severe disease, the others being thromboembolic and cardiac complications.
Nasopharyngeal swab is test of choice in which Nucleic acid amplification testing (NAAT), most commonly with a reverse-transcription polymerase chain reaction (RT-PCR) assay and Antigen tests, to detect SARS-CoV-2 RNA from the upper respiratory tract is the preferred initial diagnostic test for COVID-19. Serological testing to detect antibodies to SARS-CoV-2 can identify patients who had suffered from the infection in the recent past or within 2 weeks duration [4].
The treatment is primarily supportive with antivirals, steroids, anticoagulation and antibiotics for secondary bacterial infection. Severe cases require intensive care monitoring with oxygen support, mechanical ventilation and extra corporeal membrane oxygenation (ECMO). Investigational therapies include convalescent plasma, cytokine release inhibitors and other immunomodulatory agents like interferons. The mortality from COVID-19 appears driven by the presence of severe ARDS and is approximately 50% (range 12% to 78%) [5].
Several vaccines are under different stages of trial and include nucleic acid-based vaccines, inactivated or recombinant protein vaccines [6].
There is limited data available for COVID-19 in pregnancy as the disease has newly emerged. Most of the data came from clinical observations, case series, and case studies. There is lack of enough evidence-based data and cohort studies published to give a complete picture of the disease spectrum in pregnancy. Pregnant women have been at higher risk for severe morbidity and mortality during prior epidemic respiratory illnesses, with data from seasonal influenza, the 2009 H1N1 pandemic, and the severe acute respiratory syndrome epidemic showing higher rates of intensive care unit admission, intubation, and death compared with non-pregnant patients [7,8]. Normal physiologic changes in pregnancy, namely increased minute ventilation, reduced functional, residual capacity, upward displacement of the diaphragm, decreased chest wall compliance, and increased oxygen consumption, complicate the clinical course and management of these patients [9,10]. Additionally, maternal hypoxemia and changes to uteroplacental blood flow may cause fetal hypoxia.
SARS-CoV-1 was found to associate with high maternal morbidity and mortality during pregnancy. Adverse maternofetal outcomes like spontaneous abortion, preterm birth, and IUGR were high incidences. The mortality rate for the severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) outbreak for the general population was 10.5% and for the pregnant women was 25%. Among the latter 33% required mechanical ventilation [10]. There is little evidence available on data in pregnancy and MERS-CoV infection. However, reported cases showed a higher risk for severe pneumonia in pregnant women compared with the general population [7]. In the 1918 influenza outbreak, maternal mortality was 30% – 50% (and the mortality rate for pregnant women was twice that for nonpregnant women in the Asian influenza epidemic in the 1950s [10]. In the most recent influenza pandemic (2009 H1N1), pregnant women in the United States accounted for 6.4% of all hospitalizations and 4.3% - 5.7% of all deaths, while they typically constitute just 1% of the population [11]. The latest statistics on COVID-19 infection in pregnant women from the United States reported around 12000 cases with a mortality of 0.29% [4].
Pneumonia is a serious disease in pregnancy and requires prompt attention. Viral pneumonia has higher morbidity and mortality compared to bacterial pneumonia in pregnancy [12,13].
The inflammation driven by the virus leads to the cytokine storm profile characterized by raised levels of interleukin (IL)-2, IL-7, granulocyte-colony stimulating factor, interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumor necrosis factor-α. Viral infections and sepsis are the common causes of fulminant hyper cytokinemia leading to secondary hemophagocytic lymphohistiocytosis (sHLH), the cardinal features being unremitting fever, cytopenias, and hyperferritinemia, with lung involvement in about 50% of the cases [14].
The Inflammatory cytokine storm in COVID-19 has been strongly linked not only to the development and progression of ARDS, but also to the extra-pulmonary multiple organ failure and death [15]. Some of the early studies from China on COVID patients with ARDS treated in ICU had mortality as high as 81% and 97%. Later studies from United States and Italy showed mortality rates of 60% and 26% respectively.
While old age and hypertension were associated with higher mortality, early improvement in PaO2 to FIO2 ratio was associated with a higher chance of recovery [16].
A better characterization of COVID-19 infection in critically ill pregnant patients is important to guide decision making regarding early intervention and fetomaternal surveillance.
The aim of our case series is to describe the demographics, clinical characteristics, laboratory and radiological findings, fetomaternal outcome of severe and critical COVID pneumonia in pregnant women in Latifa Hospital. Latifa women and children hospital is a tertiary care center for maternity and pediatric services under the flagship of Dubai health authority.
This is a cross- sectional case series study of critically ill pregnant women with COVID-19 pneumonia. This was conducted at Latifa women and children hospital (LWCH), Dubai, United Arab Emirates (UAE). Latifa hospital is a 441 bedded tertiary care Maternity and Pediatric hospital. It is the main tertiary care for obstetrics and Gynecology in Dubai Emirates, it has large catchment area involves the Northern Emirates especially for the high risk cases the annual registered deliveries are 4750.
Latifa women and children hospital was recognized as a dedicated COVID care center during the pandemic, when we treated 90 cases of pregnant women with COVID, of whom seven were severe/critical infected cases admitted between January 2020 to June 2020.
Critical cases were defined as those requiring mechanical ventilation for respiratory failure or having a combined failure of other organ systems needing intensive care unit monitoring or management. All the cases were treated with anti-malarial, antivirals, steroids and anticoagulants according to the National UAE Guidelines.
We aimed to study the clinical presentation, laboratory parameters, chest X-ray findings, management and feto-maternal outcome of these cases. All cases were studied in detail after collection of their data using specific data collection sheet designed for this purpose (Table 1). The data was subsequently entered, analyzed and presented in tables and graphs.
Ethical approval
The study was approved by the Dubai Scientific Research Ethics Committee (DSREC) with approval no. DSREC-05/026. Confidentiality of the patient identity was maintained throughout the study.
| Table 1: Data Collection Tool. | |
| Demographic data | Age, BMI, Nationality |
| Obstetric history | Gravida, Parity Gestational age at presentation, Indication and mode of delivery, Postpartum status. |
| Medical history | Co-morbidities, Incubation Period, Symptoms at presentation, Clinical findings, Pao2/ Fio2 or Sao2/Fio2 ratio. |
| Laboratory investigations | CRP/PCT/FBC/ALC/RFT/LFT/D-dimer/PT/PTT/LDH/Ferritin/microbiology cultures |
| Chest X-ray | Serial Chest X-rays |
| Treatment | Antibiotics/ antivirals/antimalarials/steroids/anticoagulation/tocilizumab/ IFN/plasma exchange, Oxygen. |
| Supportive treatment | ECMO/ Dialysis/Inotropes |
| Neonatal outcome | Liquor, Birth weight, APGAR, Umbilical cord pH. |
| Neonatal COVID testing | Nasopharyngeal swab testing by RT-PCR |
| Maternal swab clearance | Number of days for swab clearance and average hospital stay |
| Maternal outcome | Stable/discharged/died. |
Patient 1
A 31-year-old, G4P3, presented at 34 weeks with fever and chills for 1 day. She had a temperature of 38.3 0C, heart rate (HR) of 120/minute, and BP of 80/45 mm Hg on clinical evaluation but maintained oxygen saturation on room air. She had Hemoglobin of 8.9% gm and platelet count of 82x109/l, Her Chest X-Ray showed involvement of less than 50% of lung fields. She was stable with the ongoing treatment, but developed cytokine storm and ARDS, and decision was taken to deliver her by cesarean section at 35 weeks. Postoperatively she was kept intubated and ventilated with inotropic support, antivirals, steroids, antibiotics, and low molecular weight heparin. She gradually showed clinical improvement with normalizing lab parameters and regression of infiltrates on chest X-ray. She was extubated on the 16th postoperative day.
Patient 2
A 28-year-old primigravida at 33 weeks of gestation, presented with a productive cough of two weeks duration with intermittent fever and flu-like symptoms for one week. On presentation, she was afebrile, normotensive, HR of 122/minute, respiratory rate (RR) of 20/minute and maintaining saturation at 95% on room air. Physical examination was unremarkable. The status of the fetus was reassuring. Chest radiography showed diffuse fluffy infiltrates. She was managed as per the hospital protocol. Patients clinical condition deteriorated with the development of cytokine storm and ARDS and she was taken for emergency cesarean section and kept ventilated and intubated. Her clinical condition improved gradually without any additional complications and she was extubated successfully after 14 days.
Patient 3
A 40-year-old Para 0+2 presented at 33 weeks of gestation with a history of shortness of breath and chest pain. She was afebrile on admission with HR 96/min, BP 110/77 mmHg, and RR 28/min with SpO2 of 98% on 15 litres O2 by Non Re- Breathable Mask NRBM. Her chest x-ray on admission showed signs of pneumonia. She was commenced on broad-spectrum antibiotics, intravenous steroids, antivirals, therapeutic thromboprophylaxis and interferon. Inspire of this, her condition worsened and she was in hypoxic respiratory failure with severe ARDS and was intubated and ventilated and taken for emergency cesarean section at 34 weeks. On the fourth postoperative day, she had a massive secondary postpartum hemorrhage, which was managed as per hospital protocol. She showed gradual improvement in her clinical symptoms, biomarkers and X-ray findings and was discharged home.
Patient 4
A 34-year-old, Para 1 at 32 weeks pregnant was admitted with complaints of shortness of breath and fever for 1 day. On admission, her temperature was 38.9, HR 152/min, RR 36/min, and was cyanosed with acute hypoxemic respiratory failure. Severe cytokine storm was suspected. She was intubated and ventilated and taken for Emergency LSCS. She was commenced on steroids, broad-spectrum antibiotics, antivirals, therapeutic anticoagulation and interferon and subsequently on inotropic support. In view of her deteriorating clinical condition, she was also treated with Interleukin-6 receptor antagonist and plasma exchange therapy.
She developed Gastro-intestinal bleed probably due to deranged coagulation profile and stress ulceration. She showed no signs of improvement and her PO2 remained 45 and PCO2 45 on 100% FiO2 with high ventilator settings. A decision to perform Veno-venous Extra-Corporeal Membrane Oxygenation (ECMO/ECLS) was done after which Saturation picked up to 97% and the Ventilator settings were reduced to Pressure control and she was gradually weaned off inotropic support. Her bedside Echo revealed Normal Ejection Fraction and left-sided Pleural Effusion. She was being maintained on ventilator support but deteriorated and developed septic shock with bilateral worsening pulmonary infiltrates and pleural effusion with persistent low minute ventilation and was restarted on inotropes, steroids, and broad-spectrum antibiotics as per hospital protocols and culture reports.
She had Secondary hemophagocytic lymph histiocytosis (sHLH), gradually became oliguric and severely acidotic with multi-organ failure had pancytopenia and thrombocytopenia with DIC, received multiple Packed Red Blood cell transfusions (PRBC) and platelet transfusions, and was on maximum inotropic support and vasopressors and required dialysis for acute kidney injury. She developed right-sided pneumothorax and the Broncho pleural fistula was on continuous drainage of the hemorrhagic left-sided pleural effusion. She gradually deteriorated with worsening hypotension, acidosis and had cardiac asystole and despite resuscitative efforts, the patient could not be revived.
Patient 5
A 34-year-old G4P3 was admitted at 29 weeks with history of high-grade fever, cough, myalgias, and breathing difficulty for 2 days. She was DCDA twin Pregnancy, conceived by in-vitro fertilization and had Gestational diabetes. On admission her HR was 116/min, RR 23/min, and SPO2 of 95% on 4-liter nasal oxygen. Her. Chest X-Ray showed evidence of pneumonia. She progressed to cytokine storm and her treatment was escalated. Peginterferon, therapeutic dose of anticoagulants, and intravenous methylprednisolone were added to her regime and she also received antenatal corticosteroids for fetal lung maturity as per protocol. She eventually developed ARDS and the decision was taken to deliver by LSCS at 30weeks 5days. Postoperatively she was kept intubated and ventilated. Cardiac evaluation and Echocardiogram were normal. She gradually showed clinical improvement with normalizing lab parameters and regression of infiltrates on chest X-ray and was extubated.
Patient 6
A 24-year-old Primigravida presented at 24 weeks of gestation with a history of fever for 9 days. On admission, her temperature was 39oC, HR 130 per minute, respiratory rate 18 per minute, blood pressure 113/58 mmHg, and SpO2 of 95% on room air. Her chest X-ray showed bilateral pulmonary infiltrates. She showed signs of clinical deterioration, cytokine storm was suspected, and she was admitted to ICU. She was started on pegylated interferon and intravenous steroids. She showed progressive improvement with noninvasive ventilation in a semi-prone position. She was discharged at 27 weeks of viable gestation in stable condition. She has safely travelled to her home country to follow up of her pregnancy.
Patient 7
A 35-year-old G2P1, presented at 7 weeks of gestation with a history of high-grade fever, cough with sputum, and shortness of breath for 1 week. Her vital signs at admission were HR- 90/minute, Blood pressure 101/84 mmmHg, SPO2 98% on 2-liters Oxygen. She was commenced on antimalarial, antiviral, azithromycin, and therapeutic anticoagulation. She was given broad-spectrum antibiotics and steroids due to persistent fever and raised septic markers. She developed high blood sugars due to steroids which were controlled by insulin. In view of worsening symptoms, raised inflammatory markers and X-ray findings, cytokine storm was suspected and one dose of pegylated interferon was given. She showed gradual improvement in her clinical condition and chest X-ray showed appreciable improvement and regression in size and density of infiltrates. She was discharged home.
In our series of cases, 5 out of seven pregnant women were in the last trimester, while 1 was in the first trimester (she is still under our antenatal care) and one was in the second trimester (she travelled back to her home country). All five women who were in the third trimester were delivered by cesarean section. Two patients had epidemiological exposure to COVID-19 related to their work. Two of them presented to us with severe COVID pneumonia. The age ranged between 28 years for the youngest patient while 40 years is the age of the oldest case (Table 2).
| Table 2: Shows the clinical characters of the patients. | |||||||
| Clinical characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
| Age (years) | 31 | 28 | 40 | 34 | 34 | 28 | 35 |
| BMI (kg/m2) | 25 | 25.39 | 29 | 31.1 | 31.2 | 32 | 30.8 |
| Gestational age at admission. | 34 weeks | 33weeks | 33 weeks | 31weeks | 29 weeks | 24 weeks | 7 weeks |
| Comorbidity | Anemia | No | DM, hypothyroid | No | GDM, Twins, IVF | No | DM |
| Onset of symptom to intubation | 8 days | 15 days | 7 days | 1 days | 9 days | NA | NA |
| Chief complaint | fever | Fever, cough | Breathing difficulty, chest pain | fever | Fever, breathing difficulty | Fever, cough | Fever, cough |
| History of exposure | Yes | Yes | No | No | No | No | No |
Two had type 2 diabetes, one had gestational diabetes and one had anemia. One diabetes patient also had hypothyroidism. Six were spontaneous singleton pregnancies and one was twin pregnancy resulted from IVF. All five delivered cases had good neonatal outcomes (well managed in ICU and discharged in good condition.) Four cases had good maternal outcomes. Unfortunately, one case was reported as maternal death (case no 4).
The main presenting symptom was fever (6/7) along with other symptoms such as cough (3/7), difficulty of breathing (2/7) and one patient had only chest pain and breathing difficulty.
Normal reference ranges in pregnancy based on normal values of the pregnant women with respect of physiological changes in pregnancy [17].
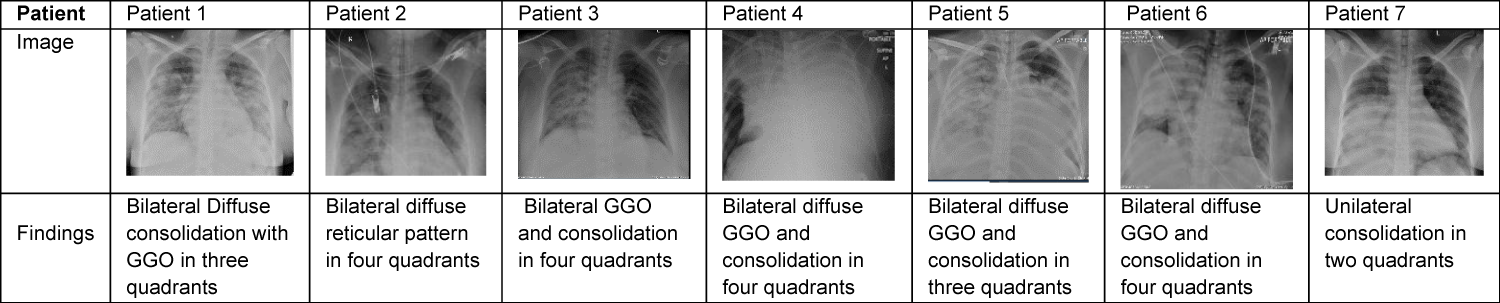
Laboratory results revealed lymphopenia for all 7 women and thrombocytopenia in one case. C Reactive protein (CRP), procalcitonin (PCT), D-Dimer, and ferritin were found to be elevated in all 7 cases. Increased aminotransferase (AST) and alanine aminotransferase (ALT) were seen in 6 patients (Tables 3,4). Chest X-rays finding went well with features of lungs decompensation and almost typical COVID presentation (Table 5).
| Table 3: Shows the laboratory results of the cases. | |||||||
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
| Hemoglobin | 10.6 (9.5-15) Normal |
11.9 (9.5-15) Normal |
13.2 (9.5-15) Normal |
12.7 (9.5-15) Normal |
9.1 (9.5-15) ↓ | 12.5 (9.7-14.8) Normal |
11.7 (11.6-13.6) Normal |
| White blood cell count (× 10⁹ cells per l) | 4.9 (5.9-16.9)↓ | 16.2 (5.9-16.9) Normal |
13 (5.9-16.9) Normal |
1.2 (5.9-16.9) ↓ | 10 (5.9-16.9) Normal |
17.3 (5.6-14.8) ↑ | 6.9 (5.7-13.6) Normal |
| Absolute Lymphocyte count (× 10⁹ cells per L) | 0.4 (<0.8) ↓ | 0.8 (<0.8) ↓ | 0.4 (<0.8) ↓ | 0.2 (<0.8) ↓ | 0.8 (<0.8) ↓ | 0.8 (<0.8) ↓ | 0.4 (<0.8) ↓ |
| Platelet count | 82 (146-429) ↓ | 356 (146-429) Normal |
168 (146-429) Normal |
347 (146-429) Normal |
220 (146-429) Normal |
179 (155-409) Normal |
312 (174-391) Normal |
| C-reactive protein concentration (mg/L) | 28 (0.4-8.1) ↑ | 107 (0.4-8.1) ↑ | 17.3 (0.4-8.1) ↑ | 341 (0.4-8.1) ↑ | 136 (0.4-8.1) ↑ | 152 (0.4-20.3) ↑ | 137 ↑ |
| Procalcitonin | 0.37(0.01-0.11) ↑ | 0.57(0.01-0.11) ↑ | 0.18(0.01-0.11) ↑ | 8.23(0.01-0.11) ↑ | 0.23(0.01-0.11) ↑ | 0.25 (0.01-0.08) ↑ | 0.62 (<0.05) ↑ |
| LDH | 314 (82-524) Normal |
325 (82-524) Normal |
1076 (82-524) ↑ | 500 (82-524) | 297 (82-524) | 567 (80-447) ↑ | 525 (70-433) ↑ |
| D Dimer | 5.1 (0.4-0.5) ↑ | 2.52 (0.4-0.5) ↑ | 2.2 (0.4-0.5) ↑ | 25.51 (0.4-0.5) ↑ | 1.95 (0.4-0.5) ↑ | 0.51 (0.2-1.6) Normal |
1.66 (0.2-0.9) ↑ |
| Ferritin | 582 (0-116) ↑ | 386 (0-116) ↑ | 3052 (0-116) ↑ | 61300 (0-116) ↑ | 145 (0-116) ↑ | 437 (2-230) ↑ | 1146 (6-130) ↑ |
| AST | 33 (4-32) ↑ | 36 (4-32) ↑ | 36 (4-32) ↑ | 45 (4-32) ↑ | 20 (4-32) Normal | 72 (3-33) ↑ | Not done |
| ALT | 9 (3-23) ↑ | 7 (3-23) ↑ | 758 (3-23) ↑ | 402 (3-23) ↑ | 14 (3-23) Normal | 127 (3-33) ↑ | 59 (3-23) ↑ |
| Patients 1, 2, 3, 4 and 5 are in third trimester; patient 6 in the second trimester and patient 7 in first trimester. | |||||||
| Table 4: Heat map from admission to discharge of the laboratory reading for 7 patients of COVID-19.ission to discharge of the 9. | |||||||||
| ID | WBC 1 | WBC 2 | WBC 3 | PLT 1 | PLT 2 | PLT 3 | HB 1 | Hb 2 | Hb 3 |
| 1 | 5 | 4.9 | 5.9 | 80 | 82 | 179 | 10.6 | 8.9 | 11.9 |
| 2 | 13.2 | 16.2 | 9 | 313 | 356 | 266 | 11.9 | 10.8 | 12.7 |
| 3 | 11.7 | 13 | 9.6 | 552 | 168 | 178 | 13.2 | 6.8 | 10.3 |
| 4 | 15.9 | 1.2 | 347 | 19 | 20 | 12.7 | 4.8 | ||
| 5 | 3.9 | 10 | 8.6 | 165 | 220 | 163 | 9.1 | 9 | 9.1 |
| 6 | 5.1 | 17.3 | 6.9 | 175 | 179 | 245 | 12.5 | 10.1 | 10.1 |
| 7 | 3.4 | 6.9 | 3.2 | 172 | 312 | 193 | 11.7 | 10.3 | 10.4 |
| ID | ALC 1 | ALC 2 | ALC 3 | CRP 1 | CRP 2 | CRP 3 | PCT 1 | PCT 2 | PCT 3 |
| 1 | 0.4 | 0.4 | 0.9 | 3.5 | 28 | 11.1 | 0.1 | 0.37 | 0.04 |
| 2 | 1.2 | 0.8 | 0.4 | 64 | 107 | 1.5 | 0.37 | 0.53 | 0.1 |
| 3 | 1.8 | 0.4 | 1 | 81.3 | 17.3 | 3.3 | 0.17 | 0.18 | 0.18 |
| 4 | 1.1 | 0.2 | 182 | 341 | 0.41 | 8.23 | 1.76 | ||
| 5 | 0.6 | 0.8 | 0.6 | 15 | 136 | 0.17 | 0.23 | 0.17 | |
| 6 | 0.8 | 0.8 | 1.7 | 55 | 152 | 17.7 | 0.09 | 0.25 | 0.08 |
| 7 | 0.7 | 0.4 | 1 | 129 | 137 | 1.4 | 0.79 | 0.62 | 0.04 |
| ID | ALT 1 | ALT 2 | ALT 3 | AST 1 | AST 2 | AST 3 | Dimer 1 | Dimer 2 | Dimer 3 |
| 1 | 9 | 9 | 29 | 0 | 33 | 18 | 0 | 5.1 | 2 |
| 2 | 19 | 7 | 14 | 36 | 36 | 13 | 1 | 2.54 | 1 |
| 3 | 28 | 758 | 203 | 36 | 36 | 126 | 1 | 2.2 | 1 |
| 4 | 11 | 402 | 135 | 32 | 45 | 3 | 25.51 | ||
| 5 | 9 | 14 | 11 | 19 | 20 | 28 | 2 | 1.95 | |
| 6 | 12 | 127 | 30 | 18 | 72 | 58 | 1 | 0.51 | 1 |
| 7 | 13 | 59 | 59 | 1 | 1.66 | 1 | |||
| ID | LDH 1 | LDH 2 | LDH 3 | Ferritin 1 | Firritin 2 | Ferritin 3 | Creatinine 1 | Creatinine 2 | Creatinine 3 |
| 1 | 0 | 314 | 233 | 125 | 582 | 336 | 0.4 | 0.4 | 0.3 |
| 2 | 342 | 325 | 344 | 210 | 386 | 360 | 0.6 | 0.4 | 0.4 |
| 3 | 345 | 1076 | 576 | 221 | 3057 | 0.5 | 0.5 | 0.3 | |
| 4 | 689 | 500 | 1033 | 46378 | 61300 | 0.6 | 1.6 | ||
| 5 | 162 | 297 | 53 | 145 | 0.3 | 0.3 | 0.3 | ||
| 6 | 187 | 567 | 235 | 106 | 437 | 0.4 | 0.4 | 0.4 | |
| 7 | 344 | 525 | 779 | 1146 | 729 | 0.5 | 0.4 | 0.4 | |
| ID | CRX 1 | CRX 2 | CRX 3 | Key: | |||||
| 1 | 2 | 4 | 0 | 1= Admission | |||||
| 2 | 4 | 4 | 0 | ||||||
| 3 | 3 | 4 | 0 | 2= Intensive care unit | |||||
| 4 | 4 | 5 | 0 | ||||||
| 5 | 3 | 4 | 0 | 3= Discharge | |||||
| 6 | 3 | 4 | 0 | ||||||
| 7 | 3 | 4 | 0 | Light blue is an abnormal and green is normal readings | |||||
Table 5: Shows the chest X- ray findings of the cases.
All patients received antiviral and antimalarial as per UAE national guideline for management of COVID-19 infections (Table 7). The choice of antibiotic for each patient was individualized according to the hospital policy, patients’ needs and culture and sensitivity reports. Six of 7 pregnant women received antimalarial medication. One patient was had ECG changes while on treatment with chloroquine. Intravenous steroids and anticoagulants were given to all 7 patients.5 patients received pegylated interferon and one patient received Tocilizumab. One patient received plasma exchange and one patient underwent ECMO.
Indication for delivery for all five patients was deteriorating maternal condition and maternal hypoxia. The mode of delivery for all the patients was LSCS for the indication of deteriorating maternal condition and maternal hypoxia.
The gestational age for delivery was between 30 weeks to 35 weeks day. The intraoperative period during the cesarean section was uneventful for all the patients. One patient had secondary postpartum hemorrhage which was managed as per hospital protocol. One patient had postpartum preeclampsia (Table 6).
| Table 6: Shows the obstetric intervention of the cases. | ||||||
| Pregnancy management | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6& 7 |
| Gestational age at delivery | 35 | 33 | 34 | 31 | 30 | Not delivered |
| Steroid for lung maturity | Not given | Received | Received | Received | Received | No yet |
| Indication for Caesarean section | Maternal hypoxia | Maternal hypoxia | Maternal hypoxia | Maternal hypoxia | Maternal hypoxia | Not yet |
| Intra op blood loss | 700ml | 600ml | 700ml | 700ml | 800ml | Not yet |
| Postoperative complications | No | Postpartum preeclampsia postpartum blues | Secondary PPH | Not yet | Not yet | Not yet |
| Table 7: Shows the drugs used for management of COVID-19 infection. |
| 1. Chloroquine Phosphate: 500 mg PO BID X 2 doses then 250 mg PO BID (Total 14- 21 days) |
| 2. Hydroxychloroquine: 400 mg PO BID X 2 doses then 200 mg PO BID (Total 14- 21 days) |
| 3. Lopinavir-Ritonavir: (200/50 mg) 2 tablets PO BID (Total 5 -7 days) |
| 4. IV Methylprednisolone: 0.5-1 mg/kg in 2 divided dose - 3 days in Non- ICU & 5-7 days in ICU |
| 5. Clexane injection: A. Therapeutic:1 mg/Kg BID for doses; B. Prophylactic: as per the body weight a. 50-90 kg: 40 mg SC. OD b. 90-130 kg: 60 mg SC. OD c. 131-170 kg: 80 mg SC. OD |
| 6. Interferon Beta 1b (Betaferon): 8 million units or 250 mcg SC on alternate days. 3 doses |
| 7. Tocilizumab: 4- 8 mg/Kg body maxiumum dose 800 mg IV single dose for cytokines storm, in partial responded case partial response second dose will be added after 8- 12 hours (maximum two doses) |
| PO: Per Oral; BID: Twice per day. IV: Intravenous. ICU: Intensive Care Unit. SC: Subcutaneous. OD: Once a Day. Either Interferon or Tocilizumab should be used |
Six live births including one twin birth were recorded. There was no fetal death, neonatal death, or serious neonatal asphyxia. All patients had premature labor but all exceeded 28 weeks of gestation. All babies had neonatal hyperbilirubinemia due to prematurity which resolved completely. All six live births had a1-min Apgar score of 7- 8 and a 5-min Apgar score of 8–9. All neonates were tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by use of real-time reverse transcriptase-polymerase chain reaction (RT-PCR) on samples from the nasopharynx once at birth and after 48 hours and resulted negative.
During the COVID outbreak, in our hospital, we managed 544 adult patients both men and women in 3 dedicated wards. Out of these 544 patients, 80 were pregnant women of which 7 became critically ill and one died.
The symptomatology and clinical findings of the seven pregnant patients in our case series with COVID-19 infection were very similar compared to non-pregnant patients with COVID-19 [18]. Similar to other COVID studies, the most common symptom was fever in our case series. Most of them had pneumonia on presentation with clinical and laboratory features of sepsis. There was no other reason other than COVID infection to explain the sepsis in these patients. The severity of pneumonia corresponded with the chest X ray findings as observed in a previous study by Daniel, et al. [18].
Cytokine storm, characterized by unremitting fever, cytopenia, hyperferritinemia were typically observed in all the patients in our series. Cytokine storm is a very expressive and descriptive term which accurately describes the severity and rapid worsening of the disease. In this series, 5 patients who were in the third trimester who had manifested cytokine storm, were delivered by cesarean section. In all these cases, severe maternal hypoxemia and maternal deterioration were the immediate reason for cesarean section. All seven patients went into a phase of severe hypoxia and rapid deterioration typically described in cytokine storm and five of them had to undergo mechanical ventilation.
The timing of delivery depends on obstetric factors and clinical deterioration. Since there is no convincing evidence of vertical transmission [19], vaginal, delivery is not contraindicated in patients with COVID-19 [20]. In the case of a critically ill parturient, cesarean section is the most appropriate mode when emergent delivery is required. Cesarean section is the preferred mode of delivery in a case of preterm pregnancy with critical COVID-19 pneumonia.
Patient 2 and patient 4 were brought in critical condition with features of the cytokine storm. They were intubated immediately (patient 2 within 12 hours and patient 3 on arrival to the emergency department) and emergency cesarean section was performed in view of severe maternal hypoxia assuming that it would improve both the maternal and fetal outcomes.
However, postoperatively neither of them showed significant improvement on account of the early delivery. On the contrary, patient 2 had a rather prolonged course of assisted ventilation. Patient 4 eventually succumbed after being on a ventilator for 39 days from complications of severe ARDS, Septic shock, and DIC despite giving pegylated interferon, plasma exchange, and ECMO. Whether the decision to perform early LSCS without adequately controlling the cytokine storm and stabilizing the patient could have contributed to the subsequent morbidity and mortality is unclear. Subjecting the patient to surgical trauma could have flared up the cytokine response [21,22].
Patient 1 and patient 2 were admitted during the early part of the COVID-19 outbreak when definite protocols were not available. They were not given steroids and anticoagulation before delivery. Both of them had prolonged ventilation and hospital stay. They also had post ICU delirium and myopathy.
Patient 3 and patient 5 were also critically ill on admission but were treated initially with therapeutic anticoagulation and steroids to manage the cytokine storm. Patient 3 was delivered on 4th day of admission and patient 5 was delivered on the 8 th day of admission. Both these patients had much less turbulent hospital course and shorter period of ventilation. The above observations clearly indicate the advantage of early treatment with steroids and anticoagulants. However, studies on a larger cohort of patients are required to confirm this observation.
Patient 6 presented in the second trimester and was promptly treated with intravenous antibiotic and therapeutic anticoagulation. Though she progressed to cytokine storm, she was adequately managed with steroids, interferon and noninvasive ventilation. She showed complete recovery.
Patient 7 presented in early first trimester with moderate pneumonia and was treated with iv antibiotic, therapeutic anticoagulation and steroids from the start. She developed cytokine storm on day 3 which was managed with interferon and noninvasive ventilation.
Patient 6 and 7 showed complete and early recovery without being ventilated probably due to the early gestational age at presentation and the aggressive mode of treatment with intravenous steroids and therapeutic anticoagulation. Probably the effect of advanced gestational age with reduced functional residual capacity, upward displacement of the diaphragm, decreased chest wall compliance, and increased oxygen consumption, complicated the clinical course and management of these patients.
Our study findings show no evidence of the vertical transmission of SARS-CoV-2. Despite prematurity, the neonatal outcome was good in this case series. In our case series, there was no incidence of severe neonatal hypoxia or neonatal death and all babies were discharged home in good condition.
Breastfeeding is not contraindicated on the basis of the current published guidance. Mothers were advised to wear face masks in order to reduce the transmission of the droplet to the new born baby. Since all of our patients were critically ill and infants were premature, infants and mothers were separated, and breastfeeding was not performed.
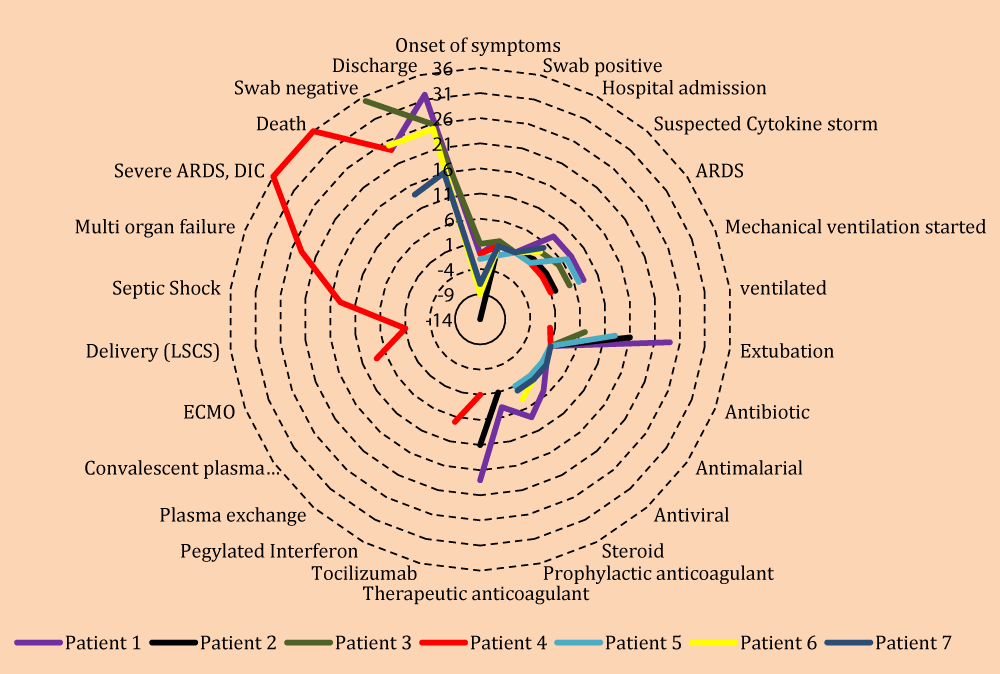
The Radar graph is very good tool for representation of such cases’ series (Figure 1).
Figure 1: Radar Graph to demonstrate multivariate variables for all seven patients.
COVID pandemic is a challenging and stressful socio-economic situation with widespread fear of infection, disease and death. A lot of effort is being put into studies and research at different levels to develop new drugs and treatment protocols to save more lives. In the specialty of obstetrics and gynecology, studies are being conducted to ascertain the manifestation of disease in pregnant women and the fetal outcome. In our series, the morbidity and mortality associated with COVID infection in pregnancy was comparable to the nonpregnant women with good fetal outcome. However, advanced gestational age could be a predictor of worse outcome. Similar to the general population, comorbidities do play a role in the outcome of disease in pregnancy also. At the end of the study, 2 things remain unclear – one, whether to deliver early in the hope if improving the chest wall compliance and lung function or to treat and stabilize the patient first before subjecting her to the stress of surgery which may flare up the cytokine response. Secondly whether to go for an early intubation and ventilation to prevent maternal and fetal hypoxia or to delay the intubation as long as possible with the aid of high flow oxygen.
COVID-19 is a newly emerged disease not well understood in this fast pandemic, medical staff yet not well expert enough especially with stress in certain protocols or guidelines which so many seen during pandemic all those mentioned and some others like psychological factors and fears with no proved vaccines yet make the constraints we faced.
- Zhu N, Zhang D, Wang W, Li X, Yang B, et al. A Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020; 382: 727-733. PubMed: https://pubmed.ncbi.nlm.nih.gov/31978945/
- World Health Organization. Novel Coronavirus—China. January 12, 2020.
- Gorbalenya AE, Baker SC, Baric R, Groot RJ, Drosten C, et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses–a statement of the Coronavirus Study Group.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323: 1239-1242. PubMed: https://pubmed.ncbi.nlm.nih.gov/32091533/
- Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020; 369. PubMed: https://pubmed.ncbi.nlm.nih.gov/32444460/
- Corey L, Mascola JR, Fauci AS, Collins FS. A strategic approach to COVID-19 vaccine R&D. Science. 2020; 368: 948-950. PubMed: https://pubmed.ncbi.nlm.nih.gov/32393526/
- Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. JAMA. 2010; 303: 1517-1525. PubMed: https://pubmed.ncbi.nlm.nih.gov/20407061/
- Lam CM, Wong SF, Leung TN, Chow KM, Yu WC, et al. A case‐controlled study comparing clinical course and outcomes of pregnant and non‐pregnant women with severe acute respiratory syndrome. BJOG: Int J Obst Gynaecol. 2004; 111: 771-774. PubMed: https://pubmed.ncbi.nlm.nih.gov/15270922/
- Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Cohen NH, et al. Miller's anesthesia e-book.: Elsevier Health Sciences; 2014.
- Metz TD. Clinical Management Guidelines for Obstetrician–Gynecologists Critical Care in Pregnancy. 2019.
- Greenberg M, Jacobziner H, Pakter J, Weisl BAG. Maternal mortality in the epidemic of Asian influenza, New York City, 1957. Am J Obstet Gynecol. 1958; 76: 897-902. PubMed: https://pubmed.ncbi.nlm.nih.gov/13583035/
- Rasmussen SA, Jamieson DJ. Coronavirus Disease 2019 (COVID-19) and Pregnancy: Responding to a Rapidly Evolving Situation. Obstet Gynecol. 2020; 135: 999-1002. PubMed: https://pubmed.ncbi.nlm.nih.gov/32213786/
- Rigby FB, PASTOREK JG. Pneumonia during pregnancy. Clin Obstet Gynecol 1996; 39: 107-119. PubMed: https://pubmed.ncbi.nlm.nih.gov/8635293/
- Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science (New York). 2020; 368: 473-474 PubMed: https://pubmed.ncbi.nlm.nih.gov/32303591/
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of the cytokine Storm' in COVID-19. J Infect 2020; 80: 607-613. PubMed: https://pubmed.ncbi.nlm.nih.gov/32283152/
- Zangrillo A, Beretta L, Scandroglio AM, Monti G, Fominskiy E, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020. PubMed: https://pubmed.ncbi.nlm.nih.gov/32353223/
- Handbook of obstetric medicine 6th edition. Catherine Nelson-Piercy 6th Edition. April 2015.
- Toussie D, Voutsinas N, Finkelstein M, Cedillo MA, Manna S, et al. Clinical and chest radiography features determine patient outcomes in young and middle-aged adults with COVID-19. Radiology. 2020; 297: E197-E206. PubMed: https://pubmed.ncbi.nlm.nih.gov/32407255/
- Chen H, Guo J, Wang C, Luo F, Yu X, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020; 395: 809-815. PubMed: https://pubmed.ncbi.nlm.nih.gov/32151335/
- Lei S, Jiang F, Su W, Chen C, Chen J, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. E Clin Med. 2020; 100331. PubMed: https://pubmed.ncbi.nlm.nih.gov/32292899/
- Paruk F, Chausse J. Monitoring the post-surgery inflammatory host response. J Emer Crit Care Med. 2019; 3.
- Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, et al. Into the eye of the cytokine storm. Microbiology and Molecular Biology Reviews. 2012; 76: 16-32. PubMed: https://pubmed.ncbi.nlm.nih.gov/22390970/
- Hirshberg A, Kern-Goldberger AR, Levine LD, Pierce-Williams R, Short WR, et al. Care of critically ill pregnant patients with coronavirus disease 2019: a case series. Am J Obstet Gynecol. 2020; 223: 286-290. PubMed: https://pubmed.ncbi.nlm.nih.gov/32371056/