More Information
Submitted: 20 November 2019 | Approved: 16 December 2019 | Published: 17 December 2019
How to cite this article: Carole AK, Felix E, Florence T, Juliette ME, Fofack TS, et al. Comparative effect of calcium supplementation on the incidence of pre-eclampsia and eclampsia among primigravid women. Clin J Obstet Gynaecol. 2019; 2: 145-149.
DOI: 10.29328/journal.cjog.1001038
Copyright License: © 2019 Carole AK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Supplementation; Calcium, Pre-eclampsia; Primigravid; Eclampsia
Comparative effect of calcium supplementation on the incidence of pre-eclampsia and eclampsia among primigravid women
Assontsa Kafack Carole1*, Essiben Felix1, Tumasang Florence1, Meka Esther Juliette1, Tongo Sedrick Fofack2 and Mbu Robinson Enow1
1Department of Obstetrics and Gynecology, Faculty of Medicine and Biomedical Science, Cameroon
2Department of Public Health, University of Abdel Nasser Conakry, Cameroon
*Address for Correspondence: Assontsa Kafack Carole, 1Department of Obstetrics and Gynecology, Faculty of Medicine and Biomedical Science, Yaounde, Cameroon, Tel: 670956463; Email: [email protected]
Background: Pre-eclampsia is a frequent and serious pregnancy complication contributing for the increasing maternal morbi-mortality rates. This study was designed to evaluate the effect of calcium supplementation during pregnancy, on the incidence of pre-eclampsia and eclampsia among primigravid women.
Method: In a hospital-based, opened, randomized and controlled clinical trial carried out in the city of Yaounde, 70 women were randomized to either 1.5 g daily calcium supplements (n = 35) or vitamins at the same time (n = 35) from 20 weeks gestation till delivery. Were included all singleton healthy, primigravid women who offered their signed inform consent and were excluded, all women with any chronic condition. Primary outcomes were pre-eclampsia and eclampsia.
Results: No significant difference was observed between the two study groups with respect to the baseline characteristics obtained at enrollment. We recorded a sevenfold decrease in the incidence of preeclampsia in the calcium group (RR = 0.26, CI 0.06 – 0.44, p = 0.024). The onset of pre-eclampsia was delayed 3 weeks in the calcium group. Meanwhile the mean diastolic blood pressure at delivery was of no significant difference (p = 0.126), the mean systolic blood pressure at delivery however, presented a significant difference between both groups (p = 0.009).
Conclusion: A 1.5 g daily calcium supplementation of healthy normotensive primigravid women during pregnancy seems to be effective in reducing the incidence of pre-eclampsia.
Pre-eclampsia is an increase in blood pressure associated with a proteinuria (≥ 300 mg/day) occurring after 20 weeks gestation in women previously known to be normotensive [1]. It is a multisystemic disorder of unknown etiology and unclear pathogenesis [2]. It is frequent among primigravid, women with diabetes, chronic hypertension, multiple pregnancy etc., [3]. Pre-eclampsia and its most deadly complication, eclampsia constitute two major components of the large clinical entity known as hypertensive disorders in pregnancy [1]. A pregnant woman is considered hypertensive if her blood pressure is greater than or equal to 140/90 mmHg on two consecutive measurements [4].
Worldwide, 10% - 15% of maternal deaths that occur every year are associated with hypertensive disorders of pregnancy (eclampsia/pre-eclampsia accounting for about 1 in 7 maternal deaths) [5,6]. It is the most prevalent maternal complication worldwide affecting about 5% – 10% of all pregnancies [1]. Pre-eclampsia and eclampsia stands out as two major causes of maternal and perinatal morbidity and mortality, affecting between 5% and 8% of all pregnancies and accounting for about 50.000 to 60.000 maternal and 500,000 fetal deaths per year worldwide [2,7].
In Africa, hypertensive disorders of pregnancy accounts for 9.1% maternal deaths and a woman’s lifetime risk of dying from pregnancy-related complications in developing countries is 14 times higher than in developed countries [8]. In sub-Saharan Africa, 1 of every 1,500 pregnancies ends in a maternal death attributable to eclampsia/pre-eclampsia [9]. In Cameroon, a prevalence of 8.2% of hypertensive disorders of pregnancy was recorded by Mboudou, et al. in 2009 (pre-eclampsia accounting for up to 77% proportion of the 4 disorders constituting this disease) [10]. The maternal mortality ratio in Cameroon has gradually been on a rise for a couple of years, from 430/100 000 live births in 1991, 669/100000 live births in 2004 to 782/100000 live births (LBs) in 2011 [11]. Nevertheless, a recent decrease was observed and recorded by the Demographic and Health Survey board in 2015 (596/100 000LBs) [12].
Several preventive measures have been proposed to decrease morbidity and mortality among pregnant women at risk of pre-eclampsia. The proposed prophylactic measures are antiplatelet therapy, magnesium sulfate and calcium supplementation [13]. Several studies in different countries, have been undertaken to study the effect of calcium supplementation on reducing the incidence of pre-eclampsia among pregnant women but conflicting results have been reported [14–20]. To the best of our knowledge, no study up to date in central Africa has evaluated this therapeutic modality.
This study was designed to evaluate the effect of calcium supplementation during pregnancy, on the incidence of pre-eclampsia and eclampsia among primigravid women.
Study population
This study was an opened, randomized controlled clinical trial performed between November 2018 and June 2019 in three hospitals in the city of Yaounde (two reference hospitals which are the Yaounde Central Hospital and the Gyneco-Obstetric and Pediatric Hospital and one district hospital; the Biyem-Assi district Hospital). Were included in this study, all singleton healthy primigravid women from 20 weeks gestation. Pregnant women already on calcium therapy or with blood pressure ≥ 140 mmHg systolic or ≥ 90mmHg diastolic, women with history of urolithiasis or with medical complicating pregnancy conditions (Diabetes Mellitus, renal failure, and heart failure) were ineligible. The gestational age was obtained from the estimates of the 1st trimester ultrasound. For estimating sample size, we used a randomized clinical trial sample size formula where type 1(α) and type 2 errors (β) were 0.05 and 0.20 (power of 80%) respectively. Accordingly we required 20 pregnant women in each group but however recruited 35 women in each group (70 participants in total) to compensate for any loss to follow-up.
Study design
We carried out a consecutive and exhaustive sampling method. All pregnant women at initial state were, randomly allocated into two groups to take either 1500 mg daily calcium supplements (as calcium carbonate) or vitamins at the same time from 20 weeks of gestation till delivery. The randomized allocation sequence was obtained from a computer-generated random number blocking (blocks of 8 women). Women in the calcium group each took three chewable tablets containing 500 mg elemental calcium giving a total of 1500 mg daily at meals, but at least 3 hours away from any iron supplements. Participants of the control group were placed on vitamins (B complexes) taken once (one tablet) daily. These medications were subsidized. Pregnant women were requested not to change their normal dietary intakes throughout the study and not to take any supplements other than that provided to them by the investigator or their physician (folic acid or ferrous sulphate). At each subsequent scheduled trial visit, they returned the boxes regardless of whether all tablets had been taken. Women were asked systematically for the tablets that were taken and encouraged to follow the treatment regimen. Individual compliance was calculated by the standard formula: 1 – (number of tablets returned/number of tablets to be taken). Treatment was discontinued when magnesium sulphate therapy was initiated to treat pre-eclampsia or when nephrolithiasis was diagnosed.
Anthropometric variables
Anthropometric measurements of the participants at enrollment were considered as baseline variables. The weight (to the nearest 0.1 kg) and height (to the nearest 0.5 cm) were measured using a scale and a stadiometer respectively. Body mass index was determined using the formula: weight (kg)/ height (m2).
Outcomes
We had as primary maternal outcome: the incidence of pre-eclampsia and/or eclampsia. Pre-eclampsia was defined as an increase in blood pressure (≥ 140/90) associated with a proteinuria or signs of target organ damage occurring after gestational week 20 [1]. Hypertension was defined as a blood pressure of ≥ 140 mm Hg and/or ≥ 90 mm Hg on 2 occasions at least 6 hours apart. Proteinuria was defined qualitatively as >2+ by dipstick, routinely performed from a urine random sample at each antenatal hospital contact or quantitatively as ≥ 300 mg/24 hours. Eclampsia was defined as a seizure in a woman with pre-eclampsia. Secondary outcomes were systolic and diastolic blood pressures at delivery and at onset of pre-eclampsia, gestational age at delivery, treatment compliance.
Blood pressure measurement and urine analysis
After about five minutes of rest, the systolic and diastolic blood pressures of all participants were measured at each subsequent visit using a standard sphygomanometer and stethoscope in sitting posture.
The first and fifth Korotkoff’s phase were recorded as systolic and diastolic blood pressures respectively. Urine samples were collected at enrollment and at each subsequent hospital visit. Midstream urine was collected in a sterile container and analyzed in less than an hour with a reactive urine dipstick with colorimetric scale. A qualitative reading of > 2+ dipstick was considered as positive for proteinuria.
Statistical analysis
Data from subjects were all included, irrespective of follow-up failures or compliance. Mean value was used as a measure of central tendency for data with normal distributions while median was used when the distribution was skewed. Distribution of data in terms of normality was evaluated by the Shapiro-Wilk test. The Student’s t - test (compare means) and the non-parametric Mann-Whitney test (compare medians) were used to detect significance for quantitative data. Categorical variables were compared using the Pearson Chi-squared test (when all cells had frequencies greater than 5) and Fisher’s exact test (when at least one cell had a frequency of less than 5).
We accessed and compared the frequency of the socio-demographic (age range, marital status, region of origin, profession, level of education) and clinical characteristics (family medical history of preeclampsia, eclampsia, hypertension, diabetes, and obesity) between both study groups. We as well calculated the mean value, standard deviation, minimum and maximum of the participant’s age, BMI, weight, height, systolic blood pressure (SBP) and diastolic blood pressure (DBP) at enrollment. We used the National Heart, Lung and Blood institute’s classification of body mass indices to group our participants into categories: the normal (18.5 - 24.9 kg/m2), overweight (25 – 29.9 kg/m2) and the obese (≥ 30 kg/m2). The group of obese were further classified by severity into classes: Class I (30 – 34.9 kg/m2), Class II (35 – 39.9 kg/m2) and Class III (≥ 40.0 kg/m2). All analysis were conducted following the approved trial protocol. Analysis were performed using SPSS version 23.0. All women provided their written informed consent and approval was obtained from the ethics committee board of the Faculty of medicine and pharmaceutical science of the University of Douala. Data analysis was done using Statistical Package for Social science (SPSS version 23.0) software. The difference was said to be statistically significant when the p value was < 0.05 and the strength of this association determined using the Risk Ratio (RR) and a 95% confidence interval.
Figure 1: The computed tomography scans in axial section of the patient. It shows a bleeding area at part of the mid brain.
Table 1 presents the baseline characteristics of the participants, Table 2 presents all pregnancy outcomes: frequency of preeclampsia, blood pressures at delivery and onset of pre-eclampsia, pregnancy duration and treatment compliance.
| Table 1: Baseline characteristics of the study participants. | |||
| Characteristics | Calcium group | Control group | p - value |
| n = 35 | n = 35 | ||
| Mean ± SD | Mean ± SD | ||
| (95% CI) | (95% CI) | ||
| Socio-demographic | |||
| Age(years) | 24.5 ± 5.8 (22.6-26.4) |
22.8 ± 4.3 (21.4-24.3) |
0.16 |
| Matrimonial status n (%) | |||
| Married women | 7 (20.0) | 11 (31.4) | 0.05 |
| Single women | 24 (68.6) | 14 (40.0) | |
| Profession n (%) | |||
| Employed | 17 (48.6) | 19 (54.3) | 0.51 |
| Level of education n (%) | |||
| Secondary | 17 (48.6) | 20 (57.1) | 0.22 |
| University | 18 (51.4) | 13 (37.1) | |
| Clinical | |||
| Family history n (%) | |||
| Hypertension | 15 (42.9) | 13 (37.1) | 0.51 |
| Diabetes | 13 (37.1) | 11 (31.4) | 0.23 |
| Height(cm) | 163.5 ± 4.7 (161.9, 165.2) |
163.7 ± 6.0 (161.7, 165.7) |
0.88 |
| Weight(kg) | 70.4 ± 9.7 (67.4, 73.7) |
72.6 ± 12.0 (68.7, 76.5) |
0.41 |
| BMI(kg/m2) | 26.5 ± 3.6 (25.3, 27.7) |
27.3 ± 4.0 (26.0, 28.7) |
0.38 |
| SBP(mmhg) | 110.2 ± 8.6 (107.3, 113.1) |
107.4 ± 12.0 (103.5, 111.3) |
0.26 |
| DBP(mmhg) | 66.9 ± 6.8 | 65.8 ± 6.9 | 0.50 |
| (64.6 69.3) | (63.7, 68.2) | ||
| BMI: Body Mass Index; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure | |||
| Table 2: Pregnancy outcomes: frequency of preeclampsia, blood pressures at delivery and onset of preeclampsia, pregnancy duration and treatment compliance. | ||||
| Secondary Outcomes | Calcium group | Control group | Absolute difference | p - value |
| (n = 35) | (n = 35) | |||
| Mean ± SD | Mean ± SD | |||
| Preeclampsia incidence | 1 (3.0%) |
7 (20.0%) |
|
0.024 |
| Mean DBP at delivery | 76.7 ± 9.4 | 80.9 ± 13.3 | 4.3 | 0.126 |
| Mean GA at onset of PE | 40 weeks ± 2 | 37 weeks ± 2 | 3.0 | 0.306 |
| Mean SBP of preeclamptic women | 134.0 ± 14.0 | 144.3 ± 14.9 | 10.3 | 0.417 |
| Mean DBP of preeclamptic women | 90.0 ± 15.0 | 96.9 ± 17.1 | 6.86 | 0.720 |
| Pregnancy duration for women with Preeclampsia (weeks) | 40.0 ± 1.3 | 38.0 ± 2.2 | 2.00 | 0.500 |
| Gestational age at delivery | 39.00 ± 1.33 | 38.74 ± 1.59 | 0.74 | 0.466 |
| Treatment compliance (%) | 75.0 (25.0 – 89.0) |
76.9 (58.4 – 90.0) |
1.92 | 0.630 |
| BMI: Body Mass Index; SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure | ||||
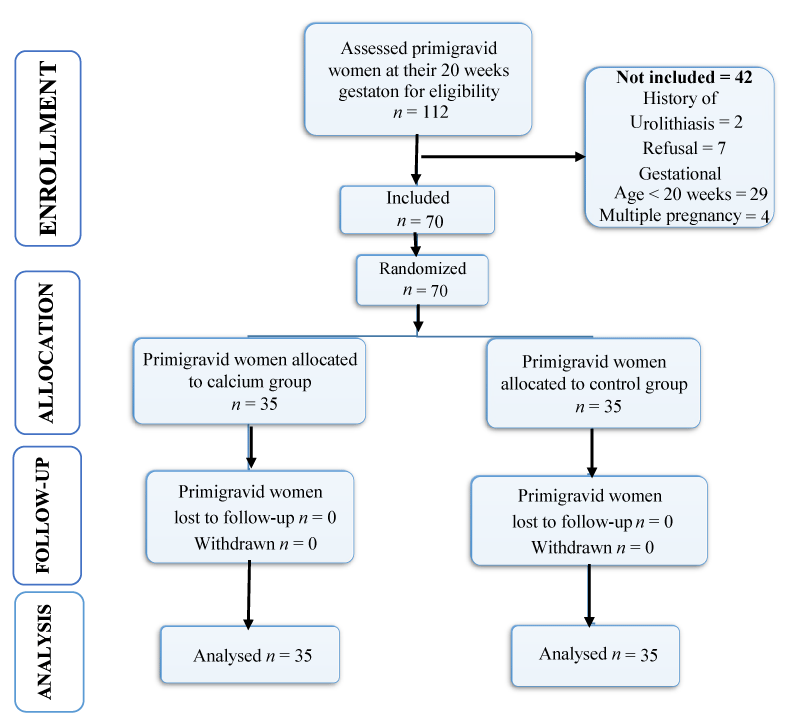
During the study, 112 women were assessed; 70 pregnant women were found to be eligible and were assigned randomly to both study groups (35 per group). No loss to follow-up was recorded in the course of the study (Figure 1). No significant difference in baseline characteristics was observed between both groups: there were no difference at randomization in age, weight, body mass index, systolic and diastolic blood pressure, level of education and family history of pre-eclampsia. Compliance to treatment was as well similar in both groups (calcium, 75%; control, 76.9%; p = 0.630).
Figure 1: Flow chart representation of recruitment of the study population.
The overall incidence of pre-eclampsia was 11.4% (8 of 70 women), and the incidence was 3.0% in the calcium group and 20.0% in the control group giving a sevenfold reduction in the occurrence of preeclampsia. A statistically significant association was found between the incidence of pre-eclampsia and the supplementation of primigravid women in calcium (p = 0.024, OR = 0.26; CI 0.06 – 0.44). None of the women in either group developed eclampsia.
The mean pregnancy duration when pre-eclampsia was detected was similar in the 2 groups. It was 40.0 ± 1.3 weeks in the calcium group and 38.0 ± 2.2 weeks in the control group (p = 0.50). The systolic and diastolic blood pressures were similar among participants who developed pre-eclampsia in the calcium (134.0 ± 14.0 mmHg and 90.0 ± 1 5.0 mm Hg) and in the control group (144.3 ± 14.9 and 96.9 ± 17.1 mm Hg). The mean duration of pregnancy in the 2 groups was similar (p = 0.466). The systolic blood pressure at delivery was significantly different between the two groups (p = 0.009).
The effect of calcium supplementation on prevention of pre-eclampsia has been a controversial issue highly debated on in several clinical trials in recent years. Majority of these trials have proven to have favorable effects for calcium supplementation during pregnancy. Calcium acts by influencing the action of calcitrophic hormones on intracellular calcium. Calcium influx in a variety of cells, including vascular smooth muscle cells (stimulated by 1,25-dihydroxyvitamin D) leads to blood pressure increase. Accordingly, low calcium diets, which elicit a 1,25-dihydroxyvitamin D response, would be expected to increase blood pressure, whereas a high diet, by virtue of suppressing 1,25-dihydroxyvitamin D levels, would be expected to reduce vascular smooth muscle cell intracellular calcium, peripheral vascular resistance and blood pressure [21]. It has as well been suggested the inhibiting role of calcium intake on parathyroid hormone release, thereby reducing renin secretion by the kidneys [14].
This study demonstrated that a daily dose of 1.5 g of calcium can reduce the occurrence of preeclampsia up to sevenfold among primigravid women. The overall incidence in pre-eclampsia was observed to be 11.4%. The difference in gestational age at supplementation and in calcium doses administered to the participants probably explains the greater incidence obtained in this study compared to that of Kumar, et al. in 2009 (7.8%) [15]. Effectively, Kumar in their study enrolled and supplemented primigravid women at 12 weeks gestation, earlier than ours (20 weeks). Also, these primigravid women in 2009 were placed on 2 g calcium, a dose higher than ours and more susceptible of having a greater protective effect on the incidence of pre-eclampsia. The black race, the average poor nutritional state and the sub-Saharan climatic condition characterizing our study population and site of study, might be contributing factors to the differences in incidences observed.
As time goes by, better management attitudes are adopted towards cases of pre-eclampsia. This might explain the absence of cases of eclampsia in this current study. To note that, even a larger sample-sized study carried over a 36 months period in recent years recorded no case as well [15]. The risk factor of eclampsia being the poor management and follow-up of participants with preeclampsia, no case of eclampsia was recorded since diagnosed pre-eclampsia cases were immediately managed.
No statistically significant difference was observed in the diastolic blood pressure at delivery (p = 0.126). A recent study carried out in Colombia by Herrera et al recorded a significant difference [22]. The inclusion of women less than 19 or greater than 35 years with a family history of preeclampsia and a daily dietary calcium intake less than 600mg might have been determining factors responsible for the significant difference observed with our recent study.
Participants in the calcium group developed pre-eclampsia 3 weeks later than the control group. The mean duration of pregnancy was 2 weeks longer in the calcium group (40.0 ± 1.3 weeks) compared to the control group (38.0 ± 2.2 weeks), findings similar to that recorded by Kumar, et al. in 2009 (39.5 ± 0.8 weeks and 37.7 ± 2.5 weeks in the calcium and control groups respectively). The observed effect could be mediated by a reduction in uterine smooth muscle contractibility induced by calcium [15].
In the light of our observation, calcium supplementation during pregnancy has proven to be effective in reducing the risk of occurrence of pre-eclampsia among primigravid pregnant women.
- Cunningham F, Kenneth J, Steven L, Jodi S, Barbara L, et al. Williams Obstetrics. 25th Edition. New York: The McGraw-Hill Companies; 706–748.
- Gathiram P, Moodley J. Pre-eclampsia: its pathogenesis and pathophysiolgy. Cardiovasc J Afr. 2016; 27: 71–78. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27213853
- Bartsch E, Medcalf KE, Park AL, Ray JG. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016; i1753. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27094586
- Yigzaw M, Zakus D, Tadesse Y, Desalegn M, Fantahun M. Paving the way for universal family planning coverage in Ethiopia: an analysis of wealth related inequality. Int J Equity Health. 2015; 14: 77. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26369946
- Ahmad A, Samuelsen S. Hypertensive disorders in pregnancy and fetal death at different gestational lengths: a population study of 2 121 371 pregnancies: Hypertensive disorder in pregnancy and fetal death. BJOG Int J Obstet Gynaecol. 2012; 119: 1521–1528. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22925135
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014; 2: e323–333.
- Kenny LC, Black MA, Poston L, Taylor R, Myers JE, et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: the Screening for Pregnancy Endpoints (SCOPE) international cohort study. Hypertension. 2014; 64: 644–652. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25122928
- World Health Organization, UNICEF, United Nations, Department of Economic and Social Affairs, Population Division, World Bank. Trends in maternal mortality: 1990 to 2015 : estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division [Internet]. 2015 [cited 2018 Dec 14]. http://www.who.int/reproductivehealth/publications/monitoring/maternal-mortality-2015/en/
- Hodgins S. Pre-eclampsia as Underlying Cause for Perinatal Deaths: Time for Action. Glob Health Sci Pract. 2015; 3: 525–527. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4682577/
- Mboudou ET, Foumane P, Priso EB, Dohbit J, Minkande JZ, et al. Hypertension in pregnancy: Clinical and epidemiologic aspects at the Yaounde Gyneco-obstetric and Pediatric Hospitalau cours de la grossesse, Cameroon. Clin Mother Child Health. 2009; 6: 1087–1093.
- Tebeu PM, Halle-Ekane G, Da Itambi M, Mbu RE, Mawamba Y, et al. Maternal mortality in Cameroon: a university teaching hospital report. Pan Afr Med J. 2015; 21: 16. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4561158/
- World Health Organization. Cooperation strategy: Health situation in Cameroon [Internet]. 2015. http://apps.who.int/gho/data/node.cco
- Jim B, Karumanchi SA. Preeclampsia: Pathogenesis, Prevention, and Long-Term Complications. Semin Nephrol. 2017; 37: 386–397. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28711078
- Aghamohammadi A, Zafari M. Calcium supplementation in pregnancy and prevention of hypertensive disorders in elderly women. Science Asia. 2015; 41: 259.
- Kumar A, Devi SG, Batra S, Singh C, Shukla DK. Calcium supplementation for the prevention of pre-eclampsia. Int J Gynaecol Obstet. 2009; 104: 32–36. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18851852
- Chen Q, Tong M, Wu M, Stone PR, Snowise S, et al. Calcium supplementation prevents endothelial cell activation: possible relevance to preeclampsia. J Hypertens. 2013; 31: 1828-1836. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23822977
- Samimi M, Kashi M, Foroozanfard F, Karamali M, Bahmani F, et al. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia. J Hum Nutr Diet. 2016; 29: 505–515. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26467311
- Shin CS, Kim KM. The Risks and Benefits of Calcium Supplementation. Endocrinol Metab. 2015; 30: 27-34. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4384676/
- Taherian AA, Taherian A, Shirvani A. Prevention of preeclampsia with low-dose aspirin or calcium supplementation. Arch Iranian Med 2002; 5: 151–156.
- Imdad A, Bhutta ZA. Effects of Calcium Supplementation during Pregnancy on Maternal, Fetal and Birth Outcomes: Calcium supplementation during pregnancy. Paediatr Perinat Epidemiol. 2012; 26: 138–152. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22742607
- Arthur TE, Sonya S, Ryan S, Rosanne H. Manual of Obstetrics. 7th Edition. Lippincott Williams and Wikins; 2007. 178–185.
- Herrera J, Arevaloherrera M, Shahabuddin A, Ersheng G, Herrera S, et al. Calcium and Conjugated Linoleic Acid Reduces Pregnancy-Induced Hypertension and Decreases Intracellular Calcium in Lymphocytes. Am J Hypertens. 2006; 19: 381–387. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16580574