More Information
Submitted: 19 November 2019 | Approved: 09 December 2019 | Published: 11 December 2019
How to cite this article: Mateshuk-Vatseba LR, Ivankiv Ya T, Ivankiv TM. The influence of opioid on the microstructural organization of the Wall OT the uterus of the white laboratory rat. Clin J Obstet Gynaecol. 2019; 2: 135-137.
DOI: 10.29328/journal.cjog.1001036
Copyright License: © 2019 Mateshuk-Vatseba LR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The influence of opioid on the microstructural organization of the Wall OT the uterus of the white laboratory rat
Mateshuk-Vatseba LR, Ivankiv Ya T and Ivankiv TM*
Department of Surgery №1, Danylo Halytsky Lviv National Medical University, Nekrasova, Lviv, Ukraine
*Address for Correspondence: Ivankiv TM, Department of Surgery №1, Danylo Halytsky Lviv National Medical University, Nekrasova, Street 4, Lviv, Ukraine 79010, Ukraine, Tel: +380 (322) 786383; Fax: +380674246699; Email: [email protected]
Drug addiction is one of the burning problems in the modern society. Annually there is a steady increase in the level of drug abuse. United Nations Office on Drugs and Crime publishes “World Drugs Report 2018”, where it was reported that about 275 million people (almost 5.6% of the world population) aged 15-64 used drugs at least once in their lives, and opium production increased by 65% in 2016-2017 [1-3]. Therefore, the question of studying the influence of drugs on the structural organization of organs remains open and relevant [4,5].
One of the main and inherent parts of our body, as a complex biological system, is the female reproductive system [6]. The female body is much more susceptible to drug addiction, and the female genital organs undergo significant structural, morphological and functional disorders [7-9]. To study the structural changes of the female reproductive system, in particular the target organ - the uterus, one of the most appropriate methods is experimental modeling [7,10,11].
Aim
To study the microstructural features of the wall of the uterus of a white laboratory rat with 4 weeks of nalbuphine exposure.
The research was performed on 12 sexually mature white female rats 3.0-3.5 months old with an initial body weight of 160-180 g. The experimental animals were divided into 2 groups: the first group of animals was injected intramuscularly with nalbuphine daily for 4 weeks (week I - 8 mg/kg, week II - 15 mg/kg, week III - 20 mg/kg, week IV - 25 mg/kg), the second group (control) was received 0.9% NaCl solution throughout the experiment.
All animals were kept in the vivarium of Danylo Halytsky Lviv National Medical University, experiments carried out in accordance with the provisions of the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes (Strasbourg, 1986), Council of Europe Directives 2010/63 /EU, Of the Law of Ukraine № 3447 - IV “On protection of animals from ill-treatment”. Materials reviewed by members of the Committee on Bioethics of Danylo Halytsky Lviv National Medical University, who agreed that the materials submitted for examination were scientifically substantiated (Protocol No. 9 of October 31, 2017).
Animals were taken out from the experiment via euthanasia by overdose of diethyl ether. Hematoxylin-eosin staining method was used for the study.
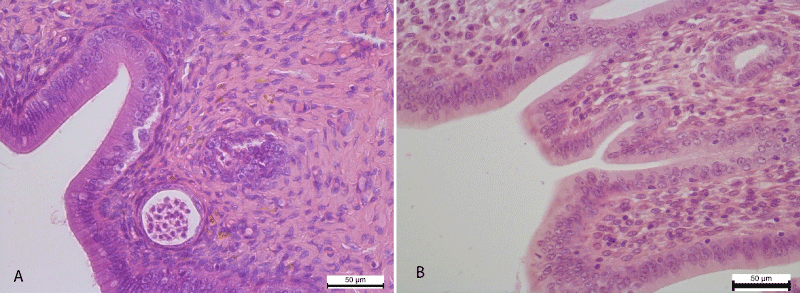
Histological examination of the uterus for 4 weeks of experimental administration of nalbuphine in the epithelium of the mucous membrane of the uterine horns recorded the development of vacuolar degeneration and necrotic changes. In particular, cytoplasm of the epitheliocytes observed cytoplasmic enlightenment and the appearance of small rounded vacuoles. Some epitheliocytes underwent necrotic changes and shelled into the lumen of the uterus. Also the development of vacuolar degeneration and necrotic changes in the epithelial cells of the uterine glands have been noticed (Figure 1).
Figure 1: A. Cytoplasmic enlightenment and small vacuoles in the cytoplasm of the epithelium of the mucous membrane of the uterine horn. Necrotic changes of the epithelium of the uterine glands. Hematoxylin and Eosin х 400. B. The mucous membrane of the uterine horn is lined with a simple columnar epithelium and deeper is the lamina of the mucous membrane. Control group. Hematoxylin and Eosin х 400.
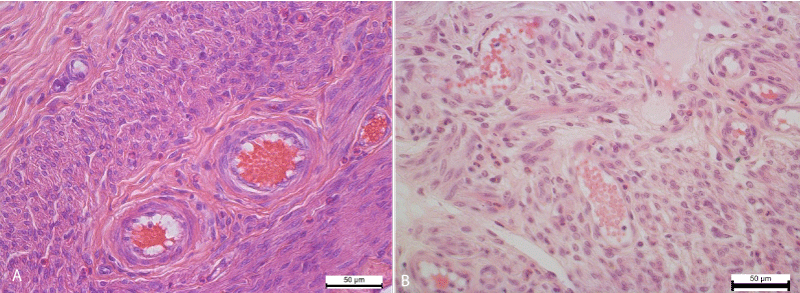
Distinct circulatory changes, in particular hyperemia and stasis, were found in the vessels of the myometrium. The small arteries and veins of the myometrium were enlarged, overflowing with erythrocytes, sometimes containing neutrophils and lymphocytes. The vessels of the hemo-microcirculatory channel were also enlarged, overflowing with erythrocytes, which are often arranged in several rows, formed figures of the coin column, glued, indicating the development of stasis. Besides the erythrocytes, neutrophils were also viewed in the lumen of the capillaries (Figure 2).
Figure 2: A. Expansion and overflow of erythrocytes of the arteries of the myometrium of the uterine horn. Hematoxylin and Eosin х 400. B. The arteries of the myometrium of the uterine horn. Control group. Hematoxylin and Eosin х 400.
Along with enlarged and overflowing veins by the erythrocytes and single neutrophils the development of hyperemia in arteries of different caliber has been noted.
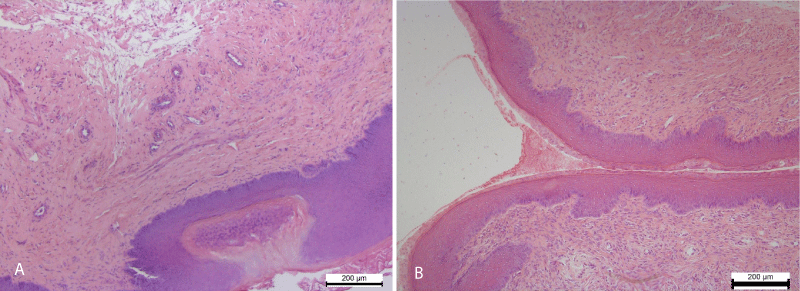
In the stratified squamous epithelium of the ectocervix was recorded Hyperplasia of the stratified squamous epithelium. The epithelial layer in areas of hyperplasia lost its architectonics and was represented by a chaotic accumulation of low-differentiated epithelial cells. In addition, in the epithelium occurred foci of necrotic epithelial changes (Figure 3).
Figure 3: A. Hyperplasia and impaired cell differentiation of the stratified squamous epithelium of the vaginal part of the cervix (ectocervix). The focus of epithelial necrosis. Necrotic changes of smooth myocyte of the myometrium. Hematoxylin and Eosin х 100. B. Stratified squamous epithelium covering the mucosa of the ectocervix (vaginal part of the cervix). A moderate amount of mucus on the surface of the mucous membrane. Control group. Hematoxylin and Eosin х 100.
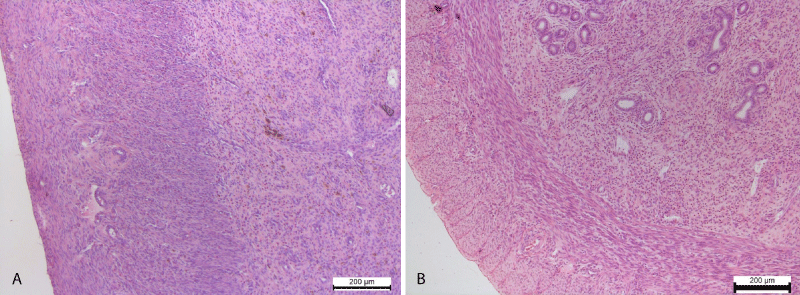
Also polymorphocellular infiltration of the myometrium was noted. Somewhere in the connective tissue of the myometrium occurred pigmentary inclusions of golden-brown color, localized in the cytoplasm of macrophages (Figure 4).
Figure 4: A. Polymorphocellular infiltration of the myometrium. Pigmentary inclusions of brown-yellow color in cytoplasm of macrophages. Hematoxylin and Eosin х 100. B. The uterine glands in its own mucous membrane. Clearly contoured smooth myocytes of myometrium. Control group. Hematoxylin and Eosin х 100.
Prolonged use of opioids causes negative changes in the female body, in particular, there are disorders in the reproductive system. In the world literature, considerable attention has been devoted to the study of opioids and pregnant uterus. In particular, some studies are described the effect of various opioids on the myometrium of pregnant female rats, where they show impaired uterine muscle contractility [9,12]. However, only a few authors describe changes in uterine structure under the influence of opioids. Maryam Dehghan, et al. [13] describe the changes they observe, namely polymorphic inflammatory infiltration with some apoptic sites and congestion of vessels, both in the myometrium and in the endometrium. Xiaofang Tang, et al. [14] in their study show that systemic administration of morphine significantly impairs proliferation and angiogenesis of the uterine stroma, thereby reducing the receptive capacity of the uterus, which ultimately impairs the implantation process.
We have not encountered in the world literature studies on the effect of nalbuphine on the structure of the uterus. We think this study is relevant to physicians, since this drug is widely used in clinical practice, and women’s reproductive health is a priority in any society.
Prolonged exposure to nalbuphine causes significant disruption of the microstructural organization of the uterine wall of the white laboratory rat. Damages detected in the experiment caused by the opioid indicates that the negative effect is capable of causing irreversible changes that lead to impaired uterine structure and function.
The data obtained indicate adverse effects of nalbuphine on women’s reproductive health, which necessitates the restriction and control of the prescription and use of narcotic analgesics in clinical practice.
- Organization WH. Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. New York: World Health Organization; 2009. PubMed: https://www.ncbi.nlm.nih.gov/books/NBK143185/
- O’Connor G, McMahon G. Complications of heroin abuse. Eur J Emerg Med. 2008; 15: 104-106. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18446076
- World Drug Report 2018. (United Nations publication, Sales No.E.18.XI.9) [Internet]. 2018. https://www.unodc.org/wdr2018.
- Center for Behavioral Health Statistics and Quality.(2015). Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50). http://www.samhsa.gov/data/
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu. Rev. Public Health 2015, 36: 559-574. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25581144
- Alimbetova AR. The study of the structure of gynecological diseases in the modern aspect. Bulletin of the Kazakh National Medical University. 2016; 1: 584-7.
- Ivankiv Ya T. Morphological features of human and white laboratory rat uterus. International scientific and practical conference. Prospects for the development of medicine in EU countries and Ukraine. Wloclawek, Republic of Poland. 2018: 21-22.
- Benningfield MM, Dietrich MS, Jones HE, Kaltenbach K, Heil SH, et al. Opioid dependence during pregnancy: relationships of anxiety and depression symptoms to treatment outcomes. Addiction. 2012; 107: 74-82. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4315620/
- Kayacan N, Ertugrul F, Arici G, Kkar M, Erman M. in vitro effect of opioids on pregnant uterine muscle. Adv Ther. 2007; 24: 368-375. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17565928
- Gartry CC, Oviedo-Joekes E, Laliberté N, Schechter MT. The trials and tribulations of implementing a heroin assisted treatment study in North America. Harm Reduction J. 2009; 6: 2. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19159475
- Onysko RM, Paltov YV, Fіk VB, Vіlkhova ІV, Kryvko Yu, et al. Method simulation of physical opioid dependence in rats. Ukrainian patent № 76564. 2013.
- Yoo KY, Lee J, Kim HS, Jeong SW. The effects of opioids on isolated human pregnant uterine muscles. Anesth Analg. 2001; 92: 1006-1009. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11273940
- Dehghan M, Jafarpour M, Mahmoudian A. The effect of morphine administration on structure and ultrastructure of uterus in pregnant mice. Iranian J Reprod Med. 2010; 8: 111-118.
- Tang X, Chen Y, Ran H, Jiang Y, He B, et al. Systemic Morphine Treatment Derails Normal Uterine Receptivity, Leading to Embryo Implantation Failure in Mice1. Bio Reprod. 2015; 92. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25855262