More Information
Submitted: 31 October 2019 | Approved: 21 November 2019 | Published: 22 November 2019
How to cite this article: Simen RCM, Vieira AA, da Cunha Ramos Miterhof MEV, de Faria AOP. Correlation between the presence of maternal gestational or pre-gestational pathologies and hearing impairment in the puerperal period. Clin J Obstet Gynaecol. 2019; 2: 122-26.
DOI: 10.29328/journal.cjog.1001033
ORCiD: orcid.org/0000-0002-4217-045X
Copyright License: © 2019 Simen RCM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hearing loss; Diabetes mellitus; Gestational diabetes; Postpartum women
Correlation between the presence of maternal gestational or pre-gestational pathologies and hearing impairment in the puerperal period
Raphaella Costa Moreira Simen1*, Alan Araújo Vieira2, Maria Elisa Vieira da Cunha Ramos Miterhof1 and Armanda de Oliveira Pache de Faria1
1Otorhinolaryngology Unit, General and Specialized Surgery Department, Fluminense Federal University, Niteroi, RJ, Brazil
2Neonatal Intensive Care Unit, Maternal and Child Department, Fluminense Federal University, Niteroi, RJ, Brazil
*Address for Correspondence: Raphaella Costa Moreira Simen, University Hospital Antonio Pedro, Fluminense Federal University (UFF), Otorhinolaryngology Unit, General and Specialized Surgery Department, Brazil, Tel: +5527992936626; Email: [email protected]
Objective: To evaluate whether the occurrence of maternal pathologies, mainly Diabetes Mellitus and Hypertensive Syndromes in the gestational or pre-gestational period may be related to hearing impairment in postpartum women.
Methods: Observational, prospective study including 361 puerperal women who had their deliveries at a reference University Hospital for pregnant women with clinical history of risk. Auditory evaluation was performed by Distortion Product Otoaccoustic Emissions (DPOAE) within 14 days after delivery. Measures of central tendency and absolute and relative frequencies were used to describe the sample and the chi-square test and binary logistic regression to assess the correlation among variables. Significance higher than 95% was observed and the study was approved by the Research Ethics Committee.
Results: A total of 361 postpartum women were studied and 7.5% had hearing impairment. The frequency of gestational hypertension was 13.9%, that of gestational diabetes was 8.6% and that of pre-pregnancy diabetes mellitus was 5.8%. The presence of hearing impairment was significantly correlated with the occurrence of pre-pregnancy diabetes mellitus (OR: 4.5 - CI: 1.51-1.47), and maternal age greater than 29 years (OR: 3.72 - 1, 58-8.76); A correlation was also found between maternal age and the presence of pre-pregnancy diabetes mellitus (OR: 3.84 - CI: 1.45-10.15).
Conclusion: In the population of postpartum women evaluated, having Diabetes Mellitus prior to pregnancy and belonging to the age group older than 29 years increases the chance of having hearing loss.
Recent studies report that the presence of maternal comorbidities may interfere in the occurrence of hearing loss in postpartum women [1-4].
It is well established in literature that hearing loss may be related to genetic predisposition, vascular causes, infections, use of ototoxic drugs, neoplastic disorders, traumatic injuries, metabolic disorders and excessive noise exposure [1-3], besides age-related hearing degeneration [5-7].
Metabolic disorders can affect cochlear physiology in several stages of the transduction of mechanical information (sound) into electrical information (neural), at least temporarily [8-10]. In the gestational period it is noteworthy the presence of high prevalence pathologies that generate characteristic metabolic disorders such as Diabetes Mellitus [11-13] and Hypertensive Syndromes [14-16], which can generate hearing impairment.
The aim of this study is to evaluate whether the occurrence of maternal diseases, especially Diabetes Mellitus (DM) and Hypertensive Syndromes (HS) during the gestational and pre-gestational periods, may be related to hearing impairment in postpartum women.
A cross-sectional study conducted at a University Hospital, a reference for the treatment of high risk pregnancy, where all mothers who had their live births performed from June 2017 until May 2018 (n = 361) were evaluated. Demographic data were collected by direct interview with the mothers and supplemented by consulting the medical records during hospitalization. All postpartum women whose auditory evaluation was performed in a postpartum period longer than 14 days were excluded from this sample.
Maternal auditory evaluation was performed using Distortion Product Otoacoustic Emissions (DPOAE) (portable equipment OtoRead SCR - Interacoustics). This instrument generates two pure tones (f1 and f2) in the stimulus intensity of 65 and 55 db SPL respectively, and four frequencies (2, 3, 4, 5 kHz) are tested. The result obtained is based on the parameters “PASS or FAIL”. A patient “passed” the test when the relationship between the signal and the background noise is equal or greater than six in three frequencies tested, thus providing an estimation of the function of the cochlear outer hair cells from this protocol of standard evaluation. Postpartum women were considered with hearing impairment when the result was “failed”. The examination was performed during hospital stay, in a silent room, at least once in each postpartum woman. If the result showed hearing impairment (failure), the test was repeated within an hour for confirmation.
The following maternal variables were evaluated: age, postpartum time on examination day, education level, skin color, quantity of prenatal consultations, gestational age, type of delivery, history of chorioamnionitis in the current pregnancy, family history of hearing loss, presence of hypertension, use of antihypertensive medication, previous or gestational diabetes mellitus, use of diabetes medication.
During hospitalization, the pressure levels and the blood glucose regulation has achieved in all patients. Unfortunately, there is no data about that during pre-natal care.
Continuous variables were described by measures of central tendency and categorical variables were described by absolute and relative frequencies. The comparison of the means between the studied groups (presence or absent of hearing impairment) was performed by the Student’s t-test when they presented normal distribution and by nonparametric tests when they did not present normal distribution.
To facilitate the assessment of the correlation between the variables, the ROC curve was used to identify the best cut-off point for the continuous variable that was significantly different between groups, transforming it into categorical variable.
The correlation between the presence of hearing impairment and the presence of comorbidities in studied groups was performed by binary logistic regression considering 5% significance level, using the statistical package SPSS 16.0 for Windows. This study was approved by the Research Ethics Committee and Informed consent was requested from all participants.
During the year when the study was conducted, a total of 437 pregnant women were hospitalized. After applying the exclusion criteria, 361 were evaluated by the study.
The predominant type of delivery was the cesarean section (69%) and only three women did not have prenatal care (0.8%). Gestational hypertension was found in 13.9% of the patients evaluated and no eclampsia was recorded as gestational complication during the study period. Previous Diabetes Mellitus was present in 5.8% postpartum women and 8.6% developed Gestational Diabetes (Table 1).
| Table 1: General characteristics of the postpartum women evaluated – continuous and categorical variables. | |||
| Mean ± SD | Median (min-max) | ||
| Mother’s age (years old) | 27.45 ± 6.72 | 27 (13-46) | |
| Number of Pre-Natal visits | 8.30 ± 2.98 | 8.0 (0-20) | |
| Gestational Age ate delivery (weeks) | 37.86 ± 2.44 | 38 (26-42) | |
| Puerperal time at examination (days) | 2.72 ± 2.06 | 2.0 (1-14) | |
| Frequency (n) | % | ||
| More than 9 school years | 288 | 79.78 | |
| Non-white | 248 | 68.7 | |
| Prenatal Care | 358 | 99.2 | |
| Cesarean Delivery | 249 | 69 | |
| Auditory impairment in any ear | 27 | 7.5 | |
| Hypertensive Diseases | Previous Hypertension Gestational Hypertension Preeclampsia Eclampsia |
50 50 70 0 |
13.9 13.9 19.4 0 |
| Diabetes Mellitus | Previous Diabetes Mellitus Gestational Diabetes |
21 31 | 5.8 8.6 |
| SD: Standart Deviation; Min: Minimum; Max: Maximum | |||
Using the DPOAE, 27 women were classified as hearing impaired. The groups were different in terms of mother’s age and the presence of previous diabetes mellitus (Table 2).
| Table 2: Comparison of puerperal women with and without hearing impairment - continuous and categorical variables. | |||
| Without hearing impairment n=334 Mean ± SD Median (min – max) |
With hearing impairment n=27 Mean ± SD Median (min – max) |
p value | |
| Mother’s Age (years old) | 27.07 ± 6.58 27 (13 – 43) | 32.19 ± 6.69 30 (20 – 46) | 0.000 |
| Prenatal visits | 8.17 ± 2.99 8 (0 – 20) | 9.85 ± 2.29 10 (5 – 14) | 0.282 |
| Gestational age at delivery (weeks) | 37.86 ± 2.43 38 (26 – 42) | 37.85 ± 2.43 38 (32 – 41) | 0.983 |
| Puerperal time at examination (days) | 2.86 ± 7.28 2 (1 – 31) | 2.89 ± 6.33 2 (1 – 14) | 0.961 |
| n (%) | n (%) | p value | |
| Previous Hypertension | 47 (14.1%) | 3 (11.1%) | 0.469 |
| Gestational Hypertension | 45 (13.5%) | 5 (18.5%) | 0.313 |
| Preeclampsia | 64 (19.2%) | 6 (22.2%) | 0.430 |
| Any Hypertensive Syndrome | 132 (39.5%) | 14 (51.9%) | 0.147 |
| Gestational Hypertension or Preeclampsia | 107 (32%) | 11 (40.7%) | 0.235 |
| Previous Diabetes Mellitus | 16 (4.8%) | 5 (18.5%) | 0.014 |
| Gestational Diabetes Mellitus | 28 (8.4%) | 3 (11.1%) | 0.415 |
| Mother’s age over 29 years | 130 (38.9%) | 19 (70.4%) | 0.001 |
| Family history of hearing loss | 12 (3.6%) | 0 (0%) | 0.388 |
| More than 9 school years | 71(21.3%) | 2 (7.4%) | 0.061 |
| Cesarean delivery | 229 (68.6%) | 20 (74.1%) | 0.360 |
| Anti-hypertensive medication in the birth period | 71 (21.3%) | 10 (37%) | 0.054 |
| Medication for Diabetes in the birth period | 38 (9.3%) | 6 (22.2%) | 0.094 |
| Use of Methyldopa during pregnancy | 93 (27.8%) | 10 (37%) | 0.210 |
| Chorioamnionitis | 8 (2.4%) | 0 (0%) | 0.534 |
| SD: Standart Deviation; Min: Minimum; Max: Maximum | |||
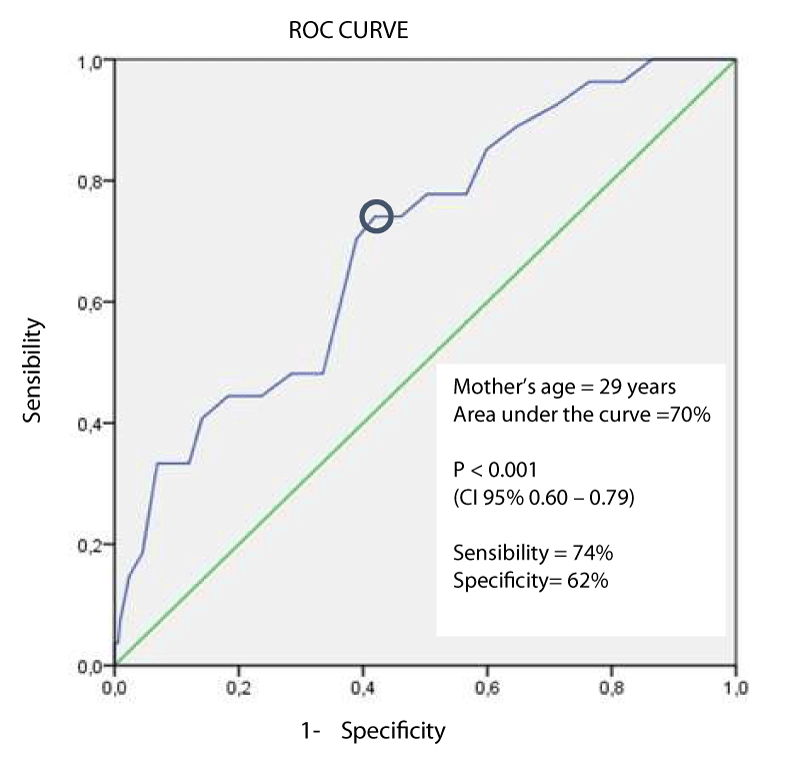
The ROC curve was constructed to determine the best cutoff point in order to categorize this variable, facilitating its analysis by logistic regression. The best cutoff point determined for mother’s age was 29 years old (Figure 1). It is important to highlight that in the postpartum population ≤ 29 years (n = 212) assessed, only 3.7% presented hearing impaired (n = 8); while in postpartum women > 29 years (n = 149), 12.8% presented this condition (n = 19).
Figure 1: ROC curve to determine the cut-off point of mother’s age in relation to the presence of hearing impairment in postpartum women.
There was no difference between the groups regarding the presence of hypertensive syndrome during or prior to pregnancy, even when they were grouped (“Total Hypertensive Syndrome” and “Gestational Hypertension or Preeclampsia”) (Table 2).
The use of anti-hypertensive drugs presented borderline difference between groups. Methyldopa was used by 95% of those women; Atenolol and Hydralazine were used only by two pregnant women and Enalapril for just one pregnant woman in this group.
The logistic regression was performed only with the variables that presented significant differences between the groups. The postpartum women over 29 years of age presented 3.7 times more chance of presenting hearing impairment and the presence of Diabetes Mellitus previous to pregnancy increased the chance of presenting hearing impairment by 4.5 times (Table 3).
| Table 3: Binary logistic regression between the presence of Diabetes Mellitus previous to pregnancy, mother’s age over 29 years and the presence of hearing impairment. | ||||
| Variable | Wald | Exp B | CI 95% | p value |
| DM previous to pregnancy | 7,308 | 4,517 | 1,514-13,478 | 0,007 |
| Mother’s age over 29 years | 9,098 | 3,727 | 1,585 – 8,762 | 0,003 |
| DM: Diabetes Mellitus; CI: Confidence Interval | ||||
When using binary logistic regression to assess the correlation between the presence of previous Diabetes Mellitus with postpartum women over 29 years an OR of 3.84 (p = 0.007 - CI95%: 1.45 – 10.15) was found.
In the group of postpartum women with hearing impairment, there was higher frequency of Diabetes Mellitus previous to pregnancy and puerperal women older than 29 years.
The correlation between Diabetes Mellitus previous to pregnancy and hearing impairment was found in the studies of Lasagni, et al. [12], Lisowska, et al. [17], and Dayem, et al. [18], who described higher frequency of hearing impairment in patients with Type 1 Diabetes, suggesting that those patients may present a sub clinic hearing disorder caused by the damage of outer hair cells, assessed by optoacoustic emission examination. Kim MB, et al. [19] in a multicenter cohort study, highlighted that the characteristic hearing loss in a group of Diabetes Mellitus patients is of sensorineural type and associated with the time of the disease and with the metabolic control of the glycaemia, evaluated by glycated hemoglobin.
In the current study, the association between hearing loss and the presence of altered glycated hemoglobin was not analyzed. However, the correlation between hearing loss, pre-gestational diabetes and maternal age greater than 29 years may be indirectly related to these factors, or rather to poor diabetes control over a prolonged period of time.
Statistic Data in Brazil [20,21] indicate that the prevalence of Diabetes Mellitus increases with advancing age. In a national survey [22], the prevalence of Diabetes Mellitus in the 18-29 age group was 0.6% and in the 30-59 age group was 5.0%. In this study, the evaluated mothers had a frequency of 5.8% of previous diagnosis of Diabetes.
Although this study did not find association between hearing impairment and gestational diabetes, Selcuk, et al. [4], found higher frequency of hearing loss in patients with gestational diabetes.
The University Hospital where the current study was conducted is a reference in the care of high risk pregnancies, mothers with hypertension and diabetes, which may justify the high prevalence of diabetes in the population studied. The incidence of gestational diabetes in the group of women studied was 8.6%, also above Brazil’s average prevalence of 7.6% [20,22].
In the present study, the frequency of women with hearing impairment was 7.5%. According to IBGE census [23], about 9.7 million Brazilians have some degree of hearing impairment, and 4.9% of Brazilian women showed some degree of hearing loss. In the analysis by age, the prevalence of hearing impairment was 4.2% in the group from 15 to 64 years. The high number of exams showing hearing impairment in the population studied may correspond to subclinical disorders, temporary cochlear alterations or may be related to the fact that the sample studied was composed of postpartum women who had a high-risk pregnancy or were referred to the hospital because of complications at the end of the pregnancy.
When we analyze the influence of age in hearing acuity, there are several reports of hearing impairment in individuals aged 45 years or older. Engdahl B, et al. [24] and Bonfils, et al. [25], described that the thresholds to obtain otoacoustic emissions linearly increase from the age of 40 years and in individuals over 60 years, only 35% presented detectable otoacoustic emission. Those results are similar to those found by Collet, et al. [26], Linssen AM, et al. [9].
The cut-off point of 29 years found in the present study reflects, mainly, the characteristic age of fertile women, i.e., it is not the most characteristic age for cochlear alterations, as presented by the authors above. However, it is noted that there is a strong correlation between being above 29 years and having Diabetes Mellitus, which may explain the higher frequency of cochlear alterations found above this age in the studied population and a possible correlation with Diabetes with poor clinical and medication control, as already mentioned above. When this correlation was analyzed, it was found that the chance of previous Diabetes Mellitus in the group above 29 years was 3.84 times higher. This result suggests that the correlations found between altered DPOAE, being older than 29 years and the presence of previous Diabetes Mellitus may reflect the interaction between these last two variables.
Another important fact to be highlighted, is that with increasing age, the prevalence of other factors that influence hearing also increases, such as exposure to noise, use of ototoxic drugs, longer evolution and severity of other possible diseases such as hypertension, autoimmune diseases, inflammatory and vascular diseases [6,27].
Regarding hypertensive diseases, in this study no association was found between the presence of previous hypertension, gestational hypertension and preeclampsia with hearing impairment. Bakhshaee, et al. [8], and Baylan, et al. [1], found a higher frequency of hearing loss in the group of women who had preeclampsia and Altuntaş, et al. [3], evaluated postpartum women with hypertensive diseases and concluded that the Transient Evoked Otoacoustic Emissions (TEOAE) was altered in the postpartum period of pregnant women with HELLP syndrome. However, in the present study, there were no cases of HELLP syndrome in the mothers evaluated.
To better elucidate the correlation between the presence of maternal diseases in the gestational period and hearing disorders, it is suggested to conduct a study with a larger and more representative sample of the general population, because the studied population has a higher incidence of specific diseases because they were attended at a reference maternity.
In the population of postpartum women evaluated in the present study, the presence of Diabetes Mellitus previous to pregnancy increased the chance of having hearing impairment by 4.5 times. Being older than 29 years increased the chance of having hearing impairment by 3.7 times. There was correlation between being older than 29 years and the presence of pre-gestational Diabetes Mellitus.
- Baylan MY, Kuyumcuoglu U, Kale A, Celik Y, Topcu I. Is preeclampsia a new risk factor for cochlear damage and hearing loss? Otol Neurotol. 2010; 31: 1180-1183. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20657326
- Oh IH, Lee JH, Park DC, Kim M, Chung JH, et al. Hearing loss as a function of aging and Diabetes Mellitus: A Cross Sectional Study. PLoS ONE. 2014; 9: e116161. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25549095
- Altuntas EE, Yenicesu AGI, Mutlu AE, Muderris S, Cetin M, et al. An evaluation of the effects of hypertension during pregnancy on postpartum hearing as measured by transient-evoked otoacoustic emissions. Acta Otorhinolaryngol Ital. 2012; 32: 31-36. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22500064
- Selcuk, Terzi H, Turkay U, Kale A, Genc S. Does gestational diabetes result in cochlear damage? J Laryngol Otol. 2014; 128: 961-965. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25399828
- Brant LJ, Gordon-Salant S, Pearson JD, Klein LL, Morrell CH, et al. Risk factors related to age-associated hearing loss in the speech frequencies. J Am Acad Audiol. 1996; 7: 152-160. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8780987
- Cilento BW, Norton SJ, Gates GA. The effects of aging and hearing loss on distortion product otoacoustic emissions. Otolaryngol Head Neck Surg. 2003; 129: 382-389. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14574293
- Abdala C, Dhar S. Maturation and Aging of the Human Cochlea: A View through the DPOAE Looking Glass. J Assoc Res Otolaryngol. 2012; 13: 403-421. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22476702
- Bakhshaee M, Hassanzadeh M, Nourizadeh N, Karimi E, Moghiman T, et al. Hearing impairment in pregnancy toxemia. Otolaryngol Head Neck Surg. 2008; 139: 298-300. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18656733
- Linssen AM, van Boxtel MP, Joore MA, Anteunis LJ. Predictors of Hearing Acuity: cross sectional and Longitudinal analysis. J Gerontol A Biol Med Sci. 2014; 69: 759-765. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24149430
- Jáuregui-Renaud K. Diabetes mellitus in the inner ear. Eur J Pharm Med Res.2016; 3: 17-22.
- Hou Y, Xiao X, Ren J, Wang Y, Zhao F. Auditory Impairment in Young Type 1 Diabetics. Arch Med Res. 2015; 46: 539-545. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26385484
- Lasagni A, Giordano P, Lacilla M, Raviolo A, Trento M, et al. Cochlear, auditory brainstem responses in Type 1 diabetes: relationship with metabolic variables and diabetic complications. Diabet Med. 2016; 33: 1260-1267. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26605750
- Jacob TA, Soares LR, Santos MR, et al. Gestacional Diabetes Mellitus: a literature review. Braz J Surg Clin Res. 2014; 6: 33-37.
- Esparza CM, Jáuregui-Renaud K, Morelos CM, Muhl GE, Mendez MN, et al. Systemic high blood pressure and inner ear dysfunction: a preliminary study. Clin Otolaryngol. 2007; 32: 173-178. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17550504
- Soares MA, Sanches SG, Matas CG, Samelli AG. The audiological profile of adults with and without hypertension. Clinics. 2016; 71: 187-192. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27166767
- Hull RH, Kerschen SR. The influence of cardiovascular health on peripheral and central auditory function in adults: a research review. Am J Audiol. 2010; 19: 9-16. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20538964
- Lisowska G, Namysowski G, Morawski K, Strojek K. Early identification of hearing impairment in patients with type 1 diabetes mellitus. Otol Neurotol. 2001; 22: 316-320. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11347633
- Dayem SM Abd El, Ghany SM Abd El, Beshr Amal E, Hassan AG, Attaya MS. Assessment of hearing in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2014; 27: 393-402. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24572980
- Kim MB, Zhang Y, Chang Y, Ryu S, Choi Y, et al. Diabetes mellitus and the incidence of hearing loss: a cohort study. Int J Epidemiol. 2017; 46: 717-726. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27818377
- Iser BPM, Stopa SR, Chueiri PS, et al. Prevalence of self-reported diabetes in Brazil: results from the National Health Survey 2013. Epidemiol Serv Saude. 2015; 24: 305-314.
- Milech A, Oliveira JEP, Vencio S, et al. Basic Principles, Evaluation and Diagnosis of Diabetes Mellitus. In Guidelines of the Brazilian Society of Diabetes (2015-2016). São Paulo; AC Farmacêutica. 2016; 1-122.
- Brazil. Ministry of Health. Health Surveillance Systems. VIGITEL: Surveillance of Risk Factors and Protection for Chronic Diseases by Telephone Survey. 2013.
- Structure by age and gender of the disabled population. IBGE 2010 Population Census - General characteristics of population, religion and persons with disabilities.
- Engdahl B. Otoacoustic emissions in the general adult population of Nord-Trondelag, Norway: I. Distributions by age, gender, and ear side. Int J Audiol. 2002; 41: 64-77. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12467372
- Bonfils P, Bertrand Y, Uziel A. Evoked Otoacoustic Emissions: normative data and presbycusis. Audiology.1988; 27: 27-35. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3377724
- Collet L, Moulin A, Gartner M, Morgon A. Age-related changes in evoked otoacoustic emissions. Ann Otol Rhinol Laryngol. 1990; 99: 993-997. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2244732
- Bittar RSM, Sanchez TG, Santoro PP, et al. Glucose metabolism and inner ear. Int Arch Otorhinolaryngology. 1998; 2: 39-42