More Information
Submitted: 05 September 2019 | Approved: 17 September 2019 | Published: 18 September 2019
How to cite this article: Korovessis P, Antonaki R, Zacharatos S, Syrimpeis V. Low back pain induces disability of women in primary uncomplicated pregnancy. Clin J Obstet Gynaecol. 2019; 2: 101-106.
DOI: 10.29328/journal.cjog.1001029
Copyright License: © 2019 Korovessis P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Primary singleton pregnancy; Low back pain; Prepartum; Postpartum; Disability; ODI; VAS
Low back pain induces disability of women in primary uncomplicated pregnancy
Panagiotis Korovessis1*, Rania Antonaki3, Spyridon Zacharatos2 and Vasileios Syrimpeis4
1MD, PhD, Orthopedic Department & Department of Gynecology and Obstrectics, General Hospital Agios Andreas, Patras, Greece
2MD, PhD, Orthopedic Spine Surgeon, Patras, Greece
3MD, Senior Gynecologist, Patras, Greece
4MD, Orthopedic Surgeon, Patras, Greece
*Address for Correspondence: Panagiotis Korovessis, MD, PhD, Orthopedic Department & Department of Gynecology and Obstrectics, General Hospital Agios Andreas, Patras, Greece, Tel: +3-6945752762; Email: [email protected]; [email protected]
Study design: A consecutive case series study
Purpose: To investigate whether Low Back Pain (LBP) in women with primary singleton pregnancy induces disability.
Background: LBP is reported to be increased in pregnants than in non-pregnant women. Different outcome measures have been used to search for correlations between pain and disability.
Methods: 167 pregnant women aged 30 ± 3.5 years participated. Two equal categorial age groups were constructed: Group A included women aged 23 - 29 years, and Group B women aged 30-39 years. Their weight was 76 ± 13 kg prepartum and the Body Mass index (BMI) was 28 ± 4 prepartum. Visual Analogue Scale (VAS) was used for LBP pain intensity and Oswestry Disability Scale (ODI) for disability estimation in the last three months prepartum and in the first three months postpartum.
Results: The women weight was 67 ± 13 kg postpartum. The BMI was 24 ± 4 postpartum. There was no difference in VAS and ODI scores versus BMI, weight and height between the two age groups in both periods of observation: prepartum and postpartum. Prepartum, 81.4% of women claimed LBP that dropped to 55.5% postpartum. ODI score dropped from 19.5 ± 13.6% prepartum to 11 ± 12% postpartum. The ODI subscales that showed significant reduction postpartum were: Pain intensity (P = 0.002); working (P = 0.009); sitting (P = 0.004); standing (P = 0.003); sleeping (P = 0.008); and traveling (P = 0.006). VAS prepartum was increasing as the weight was increasing in both periods of observation (P = 0.015 and P=0.051) respectively. VAS prepartum was significantly correlated with BMI prepartum (P = 0.019) and postpartum (P = 0.028).
Discussion: Physical disability in pregnant women was low and reduced following delivery. Disability was linked with LBP intensity, weight, BMI and height, but not with age or educational level.
Approximately 25-90% of women in their first pregnancy experience Low Back Pain (LBP) during pregnancy and after delivery [1,2]. LBP usually appears between the 20th and the 28th week of gestation and remains even 3 months postpartum [3]. The etiology of LBP in pregnancy includes hormonal, metabolic and genetic factors, increased parity, postural changes, laxity of pelvic ligaments, etc [3].
One out of three women with LBP suffer from severe pain, which reduces their quality of life or their ability to work. LBP in pregnancy is considered to be the most important risk factor for postpartum LBP and the main reason for sick leave, regarding pregnant working women [4]. Women, who have previously experienced LBP during pregnancy, experience a relapse of 85% during a subsequent pregnancy [5]. Others however reported that LBP occurs twice as often in women with a LBP history, while younger women tend to have an increased risk of LBP [5,6].
Ostgaard, et al. made the essential distinction between women who suffer from back problems before pregnancy and those who develop LBP for the first time during pregnancy with an incidence of 25-30% [5].
Eighty percent (80%) of the pregnant women with LBP claim that it affects their daily routine, while 10% of them were unable to work because of LBP [7].
LBP is often measured on with the Visual Analogue Scale (VAS). Several studies have attempted to develop a system that evaluates the extent and the effect of LBP, specifically during and after pregnancy. Some authors have used the qualified (Quebec) back pain or the Oswestry Disability Index (ODI) scales to measure the disability as the result of LBP in pregnant women. ODI, EuroQol and VAS instruments may assist in the early identification of LBP high risk pregnant women [8-10].
This paper investigates whether LBP in women with primary singleton pregnancy induces disability.
Two hundred and six (206) consecutive pregnant women, who were expected to have physiological deliver in the Department of Obstetrics and Gynecology in this Institution within one year, were initially enrolled in this longitudinal clinical study. The inclusion criteria were: a) singleton, b) primary pregnancy and c) physiological delivery. The exclusion criteria were: a) secondary or later pregnancy, b) caesarian section, d) previous spine or pelvic injury, e) spinal or iliosacral joint infection or other evidence based (CT, MRI) lumbar spine disease (history of scoliosis >10o, clinical evident lumbar/thoracolumbar kyphosis) and f) presence of any known rheumatic, endocrine or neurologic disorder.
All women were invited in two personal appointments by a senior gynecologist (second author), to complete the translated and validated in the natural women’s language ODI and VAS (0-10) questionnaire for LBP intensity. The first appointment (prepartum) was on admission for delivery and the second three months following delivery. The aim was to estimate the pregnancy associated LBP and its affection on their daily disability. ODI questionnaire includes 10 sections in total, and the maximal possible score is 50 [8,9]. For each section the total possible score is 5 (0-5): if the first statement is marked the section score is 0; if the last statement is marked, it is 5. If all the 10 sections are completed, the score is calculated as follows:
If for example the total score in a women is 16 from a possible maximal score 50, the result for the ODI score is calculated as follows: 16.50 x 100 = 32%. The minimum detectable change in ODI (90% confidence interval) is 10% points, while change < 10% may attributable to error in the measurement [8,9]. ODI scores from 0-20% stand for minimal disability; 21-40% for moderate disability; 41-60% for severe disability; 61-80% for crippled and 81-100% stand for the patients that are either bed-bound or are exaggerating for their symptoms [8,9]. In this paper the ODI scale is used to evaluate the disability status, while the VAS score is used to estimate the maximum pain severity score during the last three months prepartum and three months postpartum. The women were asked to report the highest VAS score of LBP they had during the last three months immediately prepartum and during the first trimester postpartum. Two equal categorial age groups were constructed taking the intermediate age value as basis: Group A included women aged 23-29 years, and Group B women aged 30-39 years for statistical analysis of any relation between age and other continuous variables. The women were also divided in two groups according to their education level: Group 1: Lyceum graduates and Group 2: University graduates. The continuous variables included in the analysis were: Age, weight, height, BMI, global ODI score and ODI subgroups scores and VAS score. The study was approved by the Ethics Committee at this hospital and each woman gave her informed oral & written consent.
Statistical analysis
Data were analyzed using Predictive Analytics Software (SPSS, statistics v24, Inc., Chicago, IL, USA). Continuous variables were reported as mean ± standard deviation, whereas categorial as frequencies and proportions [11].
The Skewness & Kurtosis tests were used to test the data frequency & distribution. We used the ratio Skewness: standard deviation (SD) and Kurtosis: SD to test if the extracted data are skewed or have Kurtosis; if this ratio was < 3 then we regarded as no Skewed data or Kurtosis [11]. The statistical techniques used in this study were simple descriptive techniques, graphs, paired t-test, one-way ANOVA and correlation matrices (Spearman) [11].
Correlation coefficients are used to assess the strength and direction of the linear relationships between pairs of variables. One-way ANOVA was used to compare the difference of means of continuous variables between 2 or more groups of each categorical variable [11]. Paired t-test was used to test the differences between continuous values of the same group prepartum versus postpartum.
Clinical success was defined as a ≥10% point’s improvement in pain (VAS) scores and a ≥10% point improvement in ODI [8,9,11].
One hundred and sixty-seven (81%) from the 206 pregnant women, who were initially enrolled in this study fulfilled this protocol and included in the statistical analysis. Thirty-nine (19%) women were excluded for different reasons (33 women finally received caesarean section and 6 did not completed postpartum evaluation).
The average + SD age of the 167 participants’ was 30 + 3.5 years, range 23-39 years. The women’s weight averaged 76 + 13 kg prepartum and reduced to 67 + 13 kg (paired t-test, P < 0.001) postpartum. Subsequently, there was an decrease of the average women’s BMI from 28 + 4 prepartum to 24 + 4 postpartum (paired t-test, P < 0.001) (Table 1).
| Table 1: Descriptive statistics of Anthropometric parameters and Disability (ODI) scores and frequencies | ||||
| (Min, Max) | (Mean ± SD) | Skewness | Kurtosis | |
| Age (years) | (23 - 37) | (29.65 ± 3.21) | 0.021 | 0.394 |
| Weight Prepartum (Kgr) | (59 -108) | (77.96 ± 13.59) | 0.621 | -0.388 |
| Weight Postpartum (Kgr) | (50 - 98) | (70.74 ± 12.49) | 0.575 | -0.363 |
| Height (cm) | (152 - 180) | (167 ± 7.28) | 0.056 | -0.484 |
| Education | (2* - 3**) | (2.15 ± 0.48) | 0.442 | 1.304 |
| BMI Prepartum | (22.5 - 36.5) | (27.847 ± 3.89) | 0.678 | -0.334 |
| BMI Postpartum | (19.5 - 33.1) | (25.255 ± 3.70) | 0.837 | 0.048 |
| ODI Prepartum | (0 - 48.0) | (19.478 ± 13.58) | 0.697 | -0.584 |
| ODI Postpartum | (0 - 48.0) | (10.869 ± 12.26) | 1.875 | 3.734 |
| VAS Prepartum | (0 - 9.0) | (4.173 ± 2.75) | -0.084 | -0.959 |
| VAS Postpartum | (0 - 8.0) | (1.652 ± 2.20) | 1.429 | 1.628 |
| Education level 2* = Gymnasium and 3** = University | ||||
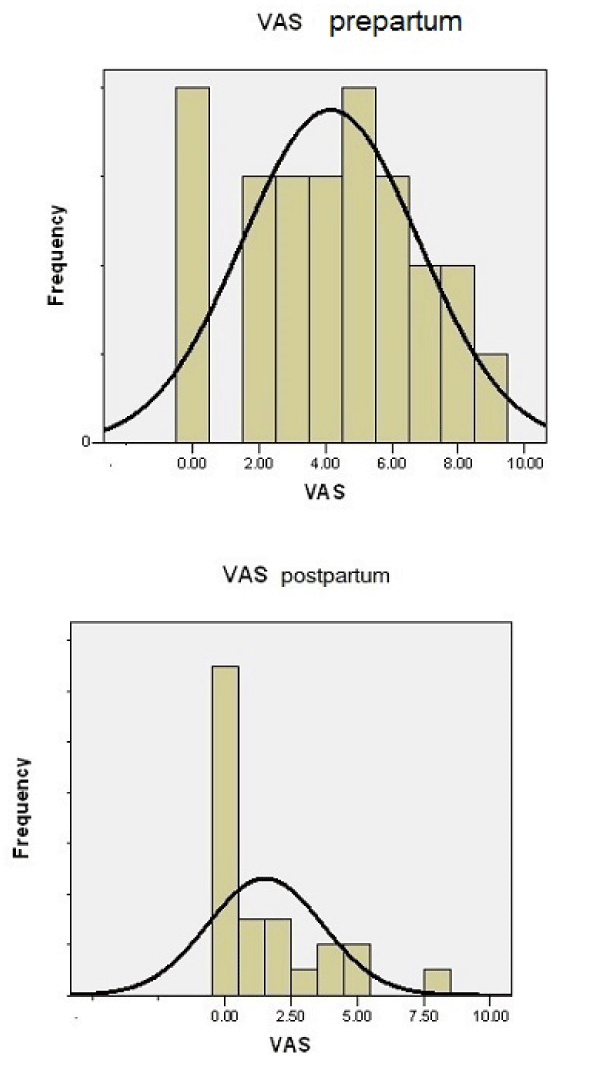
Prepartum, 27 (16%) women were pain free, while the remainder 140(84%) women claimed for some degree LBP, ranging from 1-9 (VAS) (Figure 1). In the first trimester postpartum, the number of women without pain increased to 87(52%), while 80(48%) claimed LBP (Figure 2).
Figure 1: Diagram showing the frequency of VAS prepartum and postpartum.
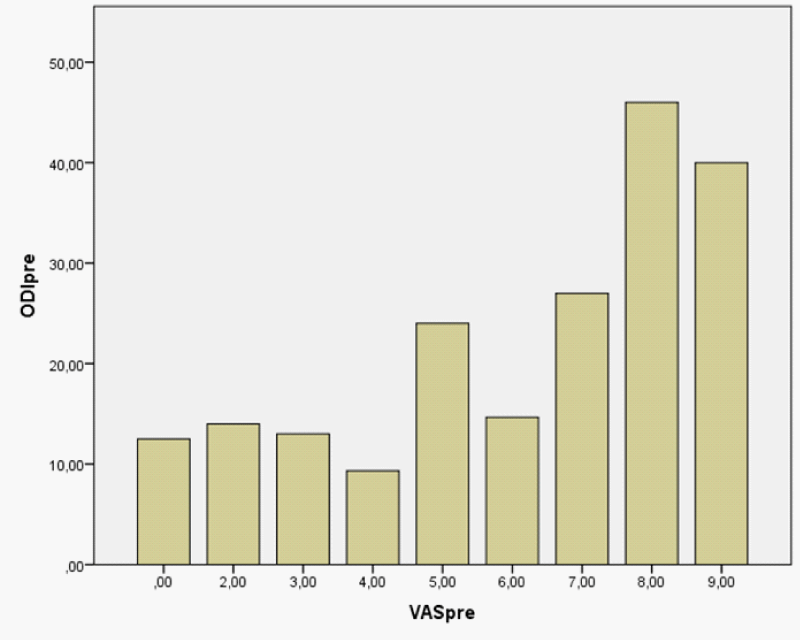
Figure 2: VAS score plotted versus ODI score prepartum.
The VAS score averaged 4.2 ± 2.8 (range 0-9) prepartum and dropped to 1.65 ± 2.2 (range 0 to 8) (paired t - test, P < 0.001) postpartum (Table 1, Figure 1). The average reduction of VAS score was 2.55 VAS grades a significant percentage redaction of 25.5% [8,9].
| Table 2: ODI sections prepartum plotted versus postpartum values (Scores 0-5 in each section). | |||
| Sections 1 - 10 | Prepartum | Postpartum | paired t - test, P - value |
| pain intensity | 1.56 ± 0.99 | 0.9 ±0.99 | 0.002 |
| Personal care | 0.30 ± 0.7 | 0.17 ± 0.65 | 0.52 |
| Lifting | 1.22 ± 1.5 | 0.91 ± 1 | 0.108 |
| Walking | 0.91 ± 0.99 | 0.47 ± 0.8 | 0.009 |
| Sitting | 1.043 ± 0.8 | 0.65 ± 0.7 | 0.004 |
| Standing | 1.52 ± 1.4 | 0.74 ± 1.2 | 0.003 |
| Sleeping | 0.60 ± 0.8 | 0.26 ± 0.69 | 0.008 |
| Sex life | 0.69 ± 1.4 | 0.39 ± 1.2 | 0.109 |
| Social life | 0.83 ± 1.15 | 0.43 ± 0.89 | 0.107 |
| Travelling | 1.043 ± 1.2 | 0.48 ± 0.9 | 0.006 |
ODI prepartum averaged 19.5 ± 13.6% (range 0 to 48%) and reduced postpartum to 10.9 ± 12.3% (range 0 to -25%), a marginal average 9.4% ODI score reduction [8,9] (Table 1).
The changes in score in each particular sections (subgroups) of the ODI questionnaire prepartum and postpartum are displaced in the table 2. There was no significant correlations between education level (secondary vs. University) and ODI (prepartum, ANOVA F = 0.196, P = 0.663 and postpartum, ANOVA F = 0.287, P = 0.599) (Figure 3).
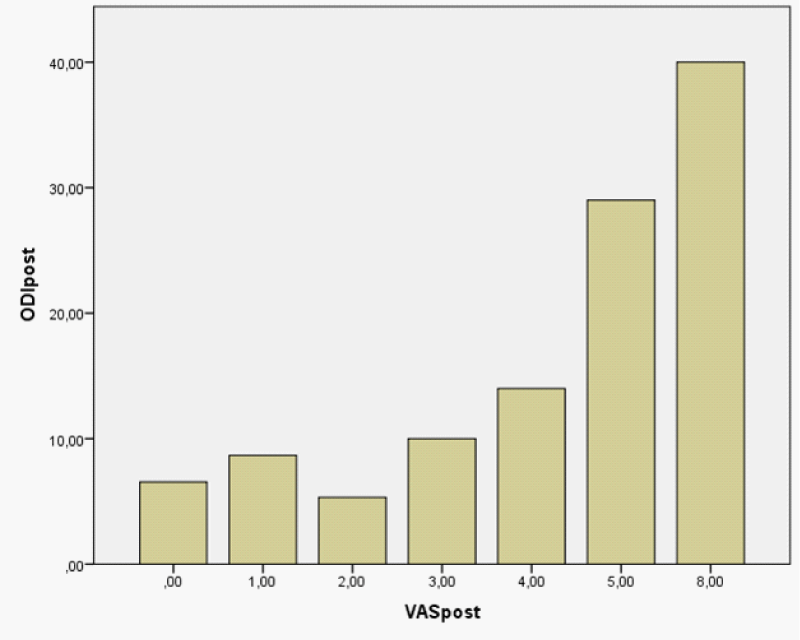
Figure 3: VAS score plotted versus ODI in postpartum.
The sections of ODI with significant reduction (improvement) were: Pain intensity (P = 0.002); walking (P = 0.009); sitting (P = 0.004); standing (P = 0.003); sleeping (P = 0.008); and traveling (P = 0.006) (Table 2).
There was no difference in VAS, ODI scores; BMI, Weight and height between the two age groups in baseline (ANOVA), (Table 3).
| Table 3: Comparison of ODI, VAS scores and Anthropometric variables between the two age Groups* (ANOVA) | ||
| F - test (ANOVA) | Significance P - value | |
| Weight Prepartum | 0.756 | 0.394 |
| Weight Postpartum | 1.067 | 0.313 |
| Height | 1.479 | 0.237 |
| BMI Prepartum | 0.168 | 0.686 |
| BMI Postpartum | 0.395 | 0.537 |
| ODI Prepartum | 0.054 | 0.818 |
| ODI Postop | 0.014 | 0.907 |
| VAS Preop | 1.976 | 0.174 |
| VAS Postop | 0.001 | 0.975 |
| *23-29 years vs 30-39 years old. | ||
VAS prepartum was significantly positively correlated with weight prepartum (Spearman R = 0.499, P = 0.015) and marginal significantly with weight postpartum (Spearman R = 0.412, P = 0.051) (Table 4).
| Table 4: Spearman Correlations. Values are presented in pairs in the form of Spearman Correlation coefficient, P-value of Significance. Correlation is significant at the level ≤0.05 & presented in BOLD. | ||||||||||
| Age | Weight Preop | Weight Postop | Height | BMI Preop | BMI Postop | ODI Preop | ODI Postop | VAS Preop | VAS Postop | |
| Age | 1 | 0.042, 0.850 | 0.124, 0.572 | 0.116, 0.597 | -0.039, 0.859 | 0.134, 0.542 | 0.194, 0.375 | 0.071, 0.749 | 0.142, 0.519 | 0.095, 0.666 |
| Weight Preop | 1 | 0.935, 0.000 | 0.682, 0.000 | 0.803, 0.000 | 0.786, 0.000 | 0.273, 0.208 | 0.254, 0.242 | 0.499, 0.015 | 0.104, 0.637 | |
| Weight Postop | 1 | 0.666, 0.001 | 0.715, 0.000 | 0.832, 0.000 | 0.181, 0.407 | 0.282, 0.192 | 0.412, 0.051 | 0.110, 0.619 | ||
| Height | 1 | 0.129, 0.558 | 0.181, 0.410 | 0.309, 0.151 | 0.421, 0.046 | 0.219, 0.317 | -0.049, 0.823 | |||
| BMI Preop | 1 | 0.898, 0.000 | 0.092, 0.676 | -0.047, 0.831 | 0.486, 0.019 | 0.158, 0.472 | ||||
| BMI Postop | 1 | 0.087, 0.693 | 0.068, 0.758 | 0.457, 0.028 | 0.223, 0.307 | |||||
| ODI Preop | 1 | 0.616, 0.002 | 0.614, 0.002 | 0.357, 0.095 | ||||||
| ODI Postop | 1 | 0.206, 0.346 | 0.444, 0.034 | |||||||
| VAS Preop | 1 | 0.405, 0.055 | ||||||||
| VAS Postop | 1 | |||||||||
VAS prepartum was significantly correlated with BMI (Spearman R = 0.486, P = 0.019), and BMI postpartum (Spearman R = 0.457, P = 0.028).
Significant correlations were shown between ODI and height postpartum (Spearman R = 0.421, P = 0.046) (Table 4).
The question posed by the authors was justified from the results retrieved in this study: pregnant women showed low physical disability (ODI) that however reduced already in the first three months following delivery. Physical disability was linked with LBP intensity, weight, BMI, height but not with women’s’ age and educational level.
More detailed, young pregnant women, who had singleton, primary pregnancy and physiological uncomplicated delivery, have a 3-month period prevalence of LBP (84%) before delivery, that however dropped to 48% within the first 3 months following delivery. This LBP pain prevalence shown in our series, seems to be higher than that reported (32.1%) in non-pregnant women in the same age range in historical series from the same geographic area and race in south-western Mediterranean country [12]. The prevalence of LBP in this series with pregnant women was considered as increased, compared to other series that reported a 9-month period prevalence of 49%, with a point prevalence of 22-28% from the 12th week until delivery [5]. In our series, pain intensity was not correlated with women’s age in both periods of observation, while in contrary others observed that younger women tend to have an increased LBP prevalence [5]. The global physical disability (ODI) in the women in our study, dropped postpartum at an average of 9.4%, that presents a marginally significant reduction.[8,9] More specifically, the sections of ODI with significant reduction postpartum were those referring women’s physical activity & pain e.g. Pain intensity (P = 0.002); working (P = 0.009); sitting (P = 0.004); standing (P = 0.003); sleeping(P = 0.008); and traveling (P = 0.006). It is impressive that sex and social life did not affect disability, as it is expressed by ODI subscale scores. Our study disclosed the expected and obvious strong correlation between ODI and VAS scores in both periods of observation, since pain level is a part of the ODI domains that induces disability.
ODI is a validated instrument available for measuring performance status or functionality in patients with lumbar spinal disease and the “gold standard” for measuring degree of disability and estimating quality of life in a person with LBP [8,9]. Our study additionally showed that the validated and national adapted ODI questionnaire, is a valuable tool to disclose even minimal disability (<20%) associated with LBP in Mediterranean pregnant women both prepartum and postpartum.
Some previous investigations disclosed that pregnancy-related LBP can evolve into chronic pain and 13.8% - 40% of women still have symptoms 3-12 months after delivery [13,14]. In our series the follow up observation was limited to 3 months postpartum since a longer follow up was not scope of this study.
In contrast to previous publications, that supported that LBP aggravates postpartum [15], in our series women reported less pain and disability postpartum than prepartum. Since pain and emotional status is a personal issue, we could speculate that this difference could be due to the different mentality of women living in a Mediterranean country. In other countries with mentality similar to ours as African and South-American, the prevalence of LBP in pregnant women ranged 25–80%, [16,17], that are close to our LBP prevalence rates.
Various mechanical explanations on the pathophysiology leading to low back & pelvic girdle pain during pregnancy and after labor have been advocated (weight gaining during pregnancy, pelvic floor dysfunction, increase of the abdominal sagittal diameter and consequent shifting of the body gravity center anteriorly, increasing the stress on the lower back) [9,18,19].
Increased BMI seems to be a risk factor for LBP in pregnancy but different reports are conflicting [20,21]. Some authors found no significant difference between BMI prepartum in those with LBP and the general population [20,21]. Our findings showed significant correlations between ODI scores & LBP, BMI, weight & height prepartum and postpartum. It seems that the less the weight and BMI of the women, the less ODI and pain scores were observed.
Further in etiology of LBP, some hormonal changes (Relaxin tenfold increase) may causing dynamic instability of the pelvis, and subsequently LBP. However, some papers support the hypothesis that the most important factor that aggravates LBP during pregnancy seems to be the progression of pregnancy [20,22,23].
Some authors consider LBP during pregnancy to be a ‘normal condition’ of pregnancy [24]. Evidently, there is a wide range in the expression of the symptoms of LBP during pregnancy, and women are affected to various degrees. Nevertheless, referring to previous literature [24,25], this condition should be considered a complication of pregnancy for women with substantial impairment. In our series the disability in pregnant women was minor and thus we cannot confirm the assumption that LBP pain during pregnancy is a complication of pregnancy.
There are two limitations in this study: 1) The LBP intensity was restricted in a period of 3 months before and 3 following delivery, in which according to the relative literature LBP appears most commonly, 2) for ethical reasons no imaging studies (CT, MRI) were made to identify the source of LBP in our series.
Most of the available studies in pregnant women assessed clinical picture, therapies and follow-up of LBP associated with pregnancy, but only few of them have assessed the severity of LBP through validated patient- oriented tools as ODI. We have performed this study on a well selected homogenous pregnant women population from a Mediterranean country using validated self-assessment questionnaires ODI and VAS, which are often used for subjective evaluation of wellbeing and pain intensity measurement. Disability in pregnant women is linked with LBP and other parameters as weight, BMI, height but not with age and educational level. LBP induced minimal physical disability that decreased subsequently in the first trimester postpartum. Reduction of weight during and after delivery and light gymnastic may contribute to LBP reduction.
- Kovacs FM, Garcia E, Royuela A, Gonzalez L, Abraira V, et al. Prevalence and factors associated with low back and pelvic girdle pain during pregnancy: a multicentre study conducted in the Spanish National Health Service. Spine. 2012; 37: 1516–1533. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22333958
- Gutke A, Josefsson A, Oberg B. Pelvic girdle pain and lumbar pain in relation to postpartum depressive symptoms. Spine. 2007; 32: 1430–1436. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17545912
- Perkins J, Hammer RL, Loubert PV.Identification and management of pregnancy-related low back pain. J Nurse Midwifery. 1998; 43: 331–340. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9803711
- Vleeming A, Albert HB, Ostgaard HC, Sturesson B, Stuge B. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008; 17: 794-819. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18259783
- Ostgaard HC, Andersson GB, Karlsson K. Prevalence of back pain in pregnancy. Spine. 1991; 16: 549-552. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1828912
- Ostgaard HC, Roos-Hansson E, Zetherstrom G. Regression of back and posterior pelvic pain after pregnancy. Spine. 1996; 21: 2777–2780. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8979325
- Vleeming A, Albert HB, Ostgaard HC, Sturesson B, Stuge B. European guidelines on the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008; 17: 794-819.
- Boscainos PJ, Sapkas G, Stilianessi E, Prouskas K, Papadakis SA. Greek versions of the Oswestry and Roland-Morris Disability Questionnaires. Clin Orthop Relat Res. 2003; 411: 40-53. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12782858
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000; 25: 2940-2952. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11074683
- Lagadec N, Steinecker M, Kapassi A, Magnier AM, Chastang J, et al. Factors influencing the quality of life of pregnant women: a systematic review. BMC Pregnancy Childbirth. 2018; 18: 455. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30470200
- Altman DG, Bland JM. Measurement in Medicine: The Analysis of Method Comparison Studies. The Statistician. 1983; 32: 307-317.
- Korovessis P, Repantis T, Zacharatos S, Baikousis A. Low back pain and sciatica prevalence and intensity reported in Mediterranean country: ordinal logistic regression analysis. Orthopedics. 2012; 35: e1775-1784. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23218636
- Gutke A, Olsson CB, Völlestad N, Öberg B, Wikmar LN, et al. Association between lumbopel¬vic pain, disability and sick leave during pregnancy - a comparison of three Scandinavian cohorts. J Rehabil Med. 2014; 46: 468-474. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24763944
- Mogren IM. BMI, pain and hyper-mobility are determinants of long-term outcome for women with low back pain and pelvic pain during pregnancy. Eur Spine J. 2006; 15: 1093-1102. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16404613
- Ostgaard HC, Zetherstrom G, Roos-Hansson E. Back pain in relation to pregnancy: a 6-year follow-up. Spine. 1997; 22: 2945-2950. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9431631
- van Dongen PW, de Boer M, Lemmens WA, Theron GB. Hypermobility and peripartum pelvic pain syndrome in pregnant South African women. Eur J Obstet Gynecol Reprod Biol. 1999; 84: 77-82. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10413232
- Duarte VM, Meucci RD, Cesar JA. Severe low back pain among pregnant women in Southern Brazil. Cien Saude Colet. 1918; 23: 2487-2494. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30137118
- Melzack R, Bélanger E. Labour pain: correlations with menstrual pain and acute low-back pain before and during pregnancy. Pain. 1989; 36: 225-229. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2521931
- Sihvonen T, Huttunen M, Makkonen M, Airaksinen O. Functional changes in back muscle activity correlate with pain intensity and prediction of low back pain during pregnancy. Arch Phys Med Rehabil. 1998; 79: 1210-1212. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9779673
- Tzeng YL, Su TJ. Low back pain during labor and related factors. J Nurs Res. 2018; 16: 231-241. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18792893
- Mens JM, Vleeming A, Stoeckart R, Stam HJ, Snijders CJ. Understanding peripartum pelvic pain. Implications of a patient survey. Spine. 1996; 12: 1363-1369. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8725930
- Sydsjo A, Sydsjo G, Wijma B. Increase in sick leave rates caused by back pain among pregnant Swedish women after amelioration of social benefits. A paradox. Spine (Phila Pa 1976). 1998; 23: 1986-1990. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9779532
- Carlson HL, Carlson NL, Pasternak BA, Balderson KD. Understanding and managing the back pain of pregnancy. Curr Womens Health Rep. 2003; 3: 65-71. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12521555
- Olsson C, Nilsson- Wilkmar L. Health-related quality of life and physical ability among pregnant women with and without back pain in late pregnancy. Acta Obstet Gynecol Scand. 2004; 83: 351-357. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15005782
- To WW, Wong MW. Factors associated with back pain symptoms in pregnancy and the persistence of pain 2 years after pregnancy. Acta Obstet Gynecol Scand. 2003; 82: 1086–1091. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14616251